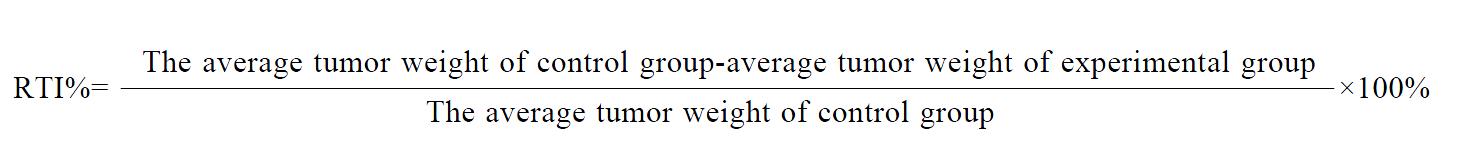Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.511
Revised: September 12, 1999
Accepted: September 29, 1999
Published online: December 15, 1999
AIM: To study the anticarcinogenic effect and acute toxicity of liver targeting mitoxantrone-nanospheres.
METHODS: The anticarcinogenic effect of mitoxantrone-polybutyl cyanoacrylate-nanoparticles ( DHAQ-PBCA-NP ) was investigated by using heterotopic and orthotopic transplantation models of human hepatocellular carcinoma ( HCC ) in nude mice and was compared with mitoxantrone (DHAQ) and doxorubicin (ADR). The acute toxicity of DHAQ-PBCA-NP lyophilized injection in mice was also studied.
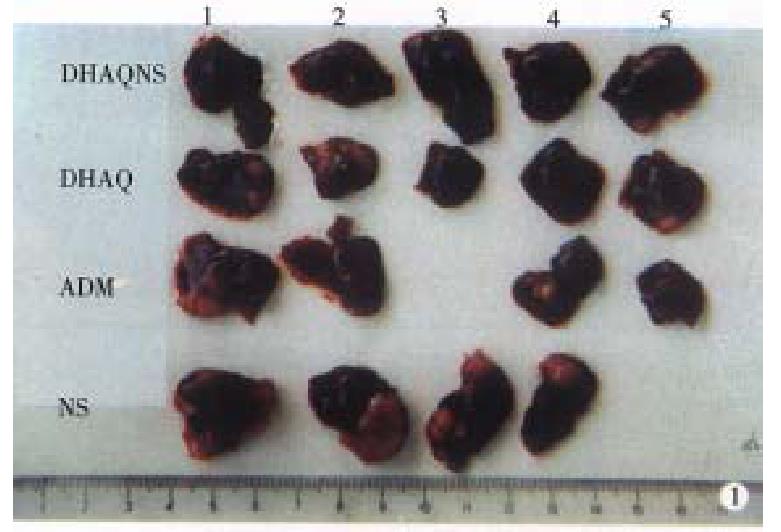
RESULTS: The tumor inhibition rates of ADR, DHAQ, DHAQ-PBCA-NP to orthotopically transplanted HCC were 60.07%, 67.49% and 99.44%, respectively, but regard to heterotopically transplanted HCC, these were 80.03%, 86.18% and 92.90%, which were concordant with the results acquired by mitosis counting and proliferating cell nuclear antigen (PCNA). After iv administration to mice with DHAQ-PBCA-NP, the LD50 was 16.9 mg/kg ± 3.9 mg/kg, no obvious local irritation was observed and there was no significant damage to the structure of liver cells, and that of the heart, spleen and kidneys.
CONCLUSION: The effect of DHAQ-PBCA-NP was significantly higher than that of DHAQ and ADR in the anti-orthotopically transplanted HCC and the acute toxicity was relatively low.
- Citation: Zhang ZR, He Q, Liao GT, Bai SH. Study on the anticarcinogenic effect and acute toxicity of liver-targeting mitoxantrone nanoparticles. World J Gastroenterol 1999; 5(6): 511-514
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/511.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.511
DHAQ is a new synthetic antitumor agent, effective in many cancers, especially in hepatic cancer, a principal cancer of high incidence and mortality[1]. Nanoparticles (NP) is a new drug carrier[3] showing a distinguished liver targeting ability, therefore, NP loading with antihepatic cancer drug could improve the effect of original drug. The DHAQ-PBCA-NP used in this study has be en proved to have remarkable liver-targeting effect. In this paper, the anticar cinogenic effect, acute toxicity and local irritation of DHAQ-PBCA-NP were studied and compared with those of ADR and DHAQ injection.
DHAQ was obtained from Organic Chemistry Department, School of Pharmacy, West China University of Medical Sciences. DHAQ-PBCA-NP was self-made with a content of DHAQ 0.15 mg/mL diameter 55.82 nm ± 12.46 nm ( n = 505 ) and drug loading 51.03%. The lyophilized ADR injection was provided by TuoBin Pharmaceutical Factory and DHAQ injection provided by Hua Da Pharmaceutical Factory with a content of DHAQ 2 mg/2 mL.
BALB/C-nu/nunude mice, Kunming mice and the heterotopic and orthotopic transplantation models of HCC in nude mice were all supplied by our Laboratory Animal Center. Animal tumor cells LTNM4 (86 generation) was obtained from Liver Cancer Laboratory, Zhongshan Hospital, Shanghai Medical University.
Tumor inhibition test of DHAQ-PBCA-NP Twenty nude mice were randomly divided into physiological saline group ( 0.1 mL/10 g ), ADR group ( 20 μg/10 g), DHAQ group ( 20 μg/10 g ) and DHAQ-PBCA-NP ( 15 μg/10 g ) group. The drug was given intravenously to each mouse 36 h after the transplantation of HCC, then given continuously once every three days for four times. On the 14th day after the last injection, the diameter of the armpit tumors of nude mice in the physiological saline group was found over 10 mm, and one mouse died. The mice were killed, and the livers as well as the tumors were taken out, weighed and the rate of tumor inhibition ( TRI ) was calculated by the following formula:
Math 1
Microscopic observations and nuclear division count of tumor The hepatic cancer of nude mice taken from control group and experimental group was sectioned into ultra-slices and observed under microscope.
Calculation of positive rate of the tumor PCNA The tumor of each groups was sampled, fixed in formalin and embedded in paraffin wax, then anti-proliferating cell nuclear antigen ( PCNA ) monoclonal antibody PC10 was used to show the proliferating cells by highly sensitive method of ABPAP, the number of tumor cells were counted and positive rate was calculated[4].
Acute toxicity One hundred and eight Kunming mice were randomly divided into DHAQ-PBCA-NP group, PBCA-NP group and DHAQ group. Each group was given 6 different dosages with the maximum dose 75.0 mg/kg, 1150 mg/kg and 25.2 mg/kg, respectively. The mice were observed for 21 d and the death rate of each group was recorded.
Pathologic examination Nine of 10 Kunming mice were given DHAQ -PBCA-NP intravenously in the dosage of 15 mg/kg and were killed after 5 min, 10 min, 15 min, 20 min, 30 min, 1 h, 24 h, 38 h and 72 h, respectively, another one was injected normal saline at the dosage of 0.1 mL/10 g. Tissue samples of the heart, liver, spleen, lung and kidney were fixed in formalin and embedded with paraffin wax for routine section, HE stain and observed under microscope. The liver tissue was fixed by glutaraldehyde, dehydrated with acetone gradually, embedded with 618 to make ultrathin section, then stained by uranium acetate and lead citrate and examined under transmission electron microscope.
Local irritation testing Ten Kunming mice were given DHAQ-PBCA -NP intravenously at a dosage of 0.1 mL/kg and the changes at the tail were observed for 1-7 days.
The tumors obtained from the liver and armpit in each group were photographed (Figures 1, 2 and 3). The tumor and tumor inhibition rate are listed in Tables 1 and 2, respectively. No significant difference was noted among the anti-HCC effect of DHAQ-PBCA-NP, DHAQ and ADR, but in the anti-orthotopically transplanted HCC, the effect by DHAQ-PBCA-NP was much higher than that by DHAQ or ADR.
| Group | Liver tumor | Armpit tumor |
| DHAQ-PBCA-NP | ||
| 1 | 0.000 | 0.215 |
| 2 | 0.000 | 0.060 |
| 3 | 0.000 | 0.005 |
| 4 | 0.000 | 0.015 |
| 5 | 0.030 | 0.015 |
| ADR | ||
| 1 | 0.820 | 0.100 |
| 2 | 0.000 | 0.050 |
| 3 | 0.675 | 0.137 |
| 4 | 0.210 | 0.410 |
| DHAQ | ||
| 1 | 0.585 | 0.270 |
| 2 | 0.650 | 0.120 |
| 3 | 0.000 | 0.065 |
| 4 | 0.000 | 0.082 |
| 5 | 0.500 | 0.066 |
| 0.9% NS | ||
| 1 | 1.320 | 1.160 |
| 2 | 1.530 | 1.026 |
| 3 | 1.000 | 0.635 |
| 4 | 0.420 | 0.670 |
| Drug | RTI (orthotopic) | RTI (heterotopic) |
| DHAQ | 67.49a | 86.18d |
| ADR | 60.07b | 80.03e |
| DHAQ-PBCA-0.9%NS | 99.44c | 92.90f |
The tumor cell proliferation in control group was very active. The tumor cell karyokinesis in DHAQ-PBCA-NP, DHAQ and ADR groups was less than that of control group (508/HP), especially in the DHAQ-PBCA-NP group, only 0-3/PH, and most were in the metaphase.
Tumor cell proliferative activity analysis The PCNA positive percentage of nude mice tumor in each group was shown in Table 3, the killing activity of DHAQ-PBCA-NP was significantly stronger than that of DHAQ and ADR (P < 0.05) on nude mice transplanted with HCC, and the activity of DHAQ was almost equal to the of ADR (P > 0.05).
| DHAQ-PBCA-NP | DHAQ | ADR | 0.9%NS | |||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| PCNA(%) | 7 | 2 | 1 | 3 | 2 | 7 | 9 | 7 | 6 | 9 | 7 | 9 | 10 | 82 | 10 | 95 | 10 | 90 |
| 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| -x±s (%) | 31.0 ± 23.0 a | 76 ± 13.4b | 85.5 ± 12.7c | 96.3 ± 4.8 | ||||||||||||||
LD50 and toxicity parameters The LD50 was calculat ed by Karber method based on the mice death rate of each dosage gorup (Table 4).
| Group | n | LD50(mg/kg, P = 0.95)* | ||
| 7 d | 14 d | 21 d | ||
| DHAQ | 60 | 12.8 ± 1.9 | 8.2 ± 1.7 | 6.1 ± 1.0 |
| DHAQ-NP | 60 | 309.9 ± 26.2 | 301.0 ± 28.3 | 299.0 ± 24.2 |
| DHAQ-PBCA-NP | 60 | 16.9 ± 3.9 | 12.3 ± 2.7 | 10.1 ± 1.9 |
The absolute lethal dose of DHAQ-PBCA-NP and DHAQ in mice was 75.0 mg/kg and 25.2 mg/kg, respectively whereas the minimal lethal dose was 6.5 and 4.8 mg/kg, respectively, the maximum tolerance dose was 4.5 mg/kg and 3.0 mg/kg, the earliest time of death was 7 d and 4 d, the latest time of death was 21 d and 16 d and the average time of death was 9.7 d ± 8.8 d and 7.5 d ± 4.2 d, respectively.
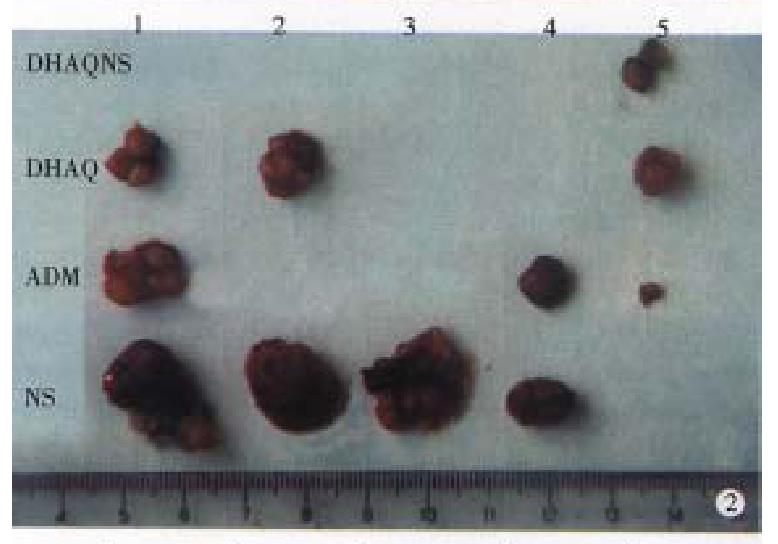
There were no apparent pathological changes in the heart, liver, spleen, lung and kidney. Under the transmission electron microscope, the liver cells were structurally intact and arranged normally, only part of the mitochondria criste were sparse and swollen (Figure 4A), but the cells returned to normal in 24 h (Figure 4B).
The tail veins of the mice were stained blue 1-7 days after i.v. DH AQ-PBCA-NP. Except one which appeared red and swollen sightly, the stained blue disappeared completely 4 days later, which demonstrated no irritation by DHAQ-PBCA-NP injection.
The effect of DHAQ-PBCA-NP on the anti-heterotopically transplanted HCC was almost the same as that of DHAQ and ADR, which implied that it was more eligible to use the model of orthotopic transplantation than to use the heterotopic one.
The cell proliferative activity could be used for assessing the efficacy of chemotherapy. PCNA was an antigen existed largely at the junction of G1 phase and S phase in the process of tumor cell proliferation, PC10 was the monoclonal antibody of PCNA. In order to investigate inhibiting effect of various drugs on tumor cell proliferation, a highly sensitive method of ABPAP was used for expression of PCNA by PC10 in this study, and the results were satisfactory. Furthermore, the DHAQ-PBCA-NP injection had no adverse effect on the structure of heart, spleen, lung and kidney only some mild and reversible changes on the mitochondria of the hepatocytes implicating preparation of DHAQ-PBCA-NP was more effective and much less toxic than DHAQ.
Edited by Xie-Ning Wu
Proofread by Qi-Hong Miao
| 1. | Gu GW, Lu SZ. Hepatocarcinoma pathological epidemiology. For-eign Med Sci-Physiol, Pathol Clin Fascicle. 1991;11:91-93. |
| 2. | Ma YP, Zheng S. The anticancer drug mitoxantrone. World Pharm-Synthet Drug. Biochem Drug Pharmac Fascicle. 1986;7:324-327. |
| 3. | Couvreur P, Kante B, Roland M, Guiot P, Bauduin P, Speiser P. Polycyanoacrylate nanocapsules as potential lysosomotropic carriers: preparation, morphological and sorptive properties. J Pharm Pharmacol. 1979;31:331-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 285] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Garcia RL, Coltrera MD, Gown AM. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989;134:733-739. [PubMed] |