Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.506
Revised: September 6, 1999
Accepted: September 22, 1999
Published online: December 15, 1999
AIM: To improve the technique of intraportal embolization (PVE) therapy, a new embolic method, was devised and the safety, effectiveness and feasibility were evaluated.
METHODS: PVE with intraportal ethanol injection via a fine needl e was performed in 28 normal dogs, 22 SD rats, and 24 cirrhotic SD rats. After P VE, portography, histological and functional alteration of the liver were evaluated in dogs and rats, and the changes in portal hemodynamics as well as hepatic anatomy were observed in rats. In the clinical study, PVE by ethanol injection was performed in 61 patients with hepatocellular carcinoma under the guidance of portoechography with intraportal injection of CO2. The effect of PVE was evaluated by ultrasonography and laparotomy.
RESULTS: The effectiveness and toxicity were dependent on the do se of ethanol. In the dogs, 0.25 mg/kg of ethanol caused incomplete embolization with least liver damage, while 1.0 mg/kg induced complete embolization with a high mortality of 57.1% (4/7) due to respiratory arrest. The dose of 0.5 mg/kg resulted in complete embolization with slight toxicity to the liver. In the rats, the survival rate was 100% in normal group but 40.9% in cirrhotic models after ethanol injection by dose of 0.05 mg/100 g. PVE for cirrhotic rats with 0.03 mg/100 g of ethanol induced satisfactory embolization with significant hypertrophy in nonembolized lobes, and only slight damage to the hepatic parenchyma, and transient alteration in liver function, portal pressure and portal flow. In the clinical study, 12 cases with reverse portal flow were excluded judged by portoechography. Satisfactory embolization was gained in 90.2% (55/61) of the remaining patients determined by ultrasonography and surgery. All cases ran an uneventful postembolization course with no aberrant embolization.
CONCLUSION: PVE with intraportal ethanol injection of appropriate dosage via a fine needle is safe and effective and has several advantages comparing with transcatheter method. Portoechography is a mandatory approach for the prevention of aberrant embolization.
- Citation: Lu MD, Chen JW, Xie XY, Liang LJ, Huang JF. Portal vein embolization by fine needle ethanol injection: experimental and clinical studies. World J Gastroenterol 1999; 5(6): 506-510
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/506.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.506
Hepatocellular carcinoma (HCC) is one of the most common malignancies in China. The resection rate is less than 30% since most patients are associated with cirrhosis and poor liver function. Furthermore, there are tumor emboli in the portal vein which limits surgical resection. Portal vein embolization (PVE) has been performed to increase the safety and resectability of hepatectomy by improving the functional reserve of the liver[1-3], and prevent cancerous dissemination via portal vein[4]and enhance the therapeutic efficacy of transcatheter arterial chemoembolization (TACE)[5]. However, conventional PVE requires catheterization under both sonographic and fluoroscopic guidance, and selective embolization is not easy. To improve the technique, we developed a met hod of intraportal ethanol injection via a fine needle under the guidance of portoechography. This study is mainly to investigate the safety, effectiveness and feasibility of this technique and a series of experimental and clinical studies were carried out.
Twenty-eight mongrel dogs of both sexes weighing 7.5 kg-15.0 kg we re provided from the Laboratory Animal Center of our university. Under intraper itoneal anesthesia with sodium pentobarbital 30 mg/kg, laparotomy was per formed and the left portal branches were exposed. Puncture of the origin of the portal branches supplying the left central and lateral lobes was done with a 22-gauge needle. Then 95% ethanol was injected at a dose of 0.25 mL/kg (group A, n = 7), 0.5 mL/kg (group B, n = 11) and 1.0 mL/kg (group C, n = 7) at a rate of 3 mL/min.
One or two dogs in each group underwent relaparotomy 30 or 60 min after injection and a 6 Fr catheter was placed in the portal trunk. The liver together with the portal trunk was taken out. Portography with the mixture of Urografin (Schering AG, Germany) and Lipiodol (Guerbet, France) via the catheter was performed. Then the intrahepatic portal system was dissected to confirm the site of the thrombus. The same procedures were carried out at day 1 and 3, as well as of week 1, 2 or 4 in groups A and B and week 2 or 4 in group C after ethanol embolization. Tissue samples from embolic and nonembolic liver lobes were examined histologically at the same time.
Sprague-Dawley (SD) rats with a body weight between 200 g-250 g were used in this experiment. Initially, twenty normal rats had liver resected under a nesthesia, and the right, middle, left lobes and the whole liver of each rat were weighed. The mean weight ratios of right, middle and left lobes to the whole liver were 40.5%, 36.5% and 23.0%, respectively.
The cirrhotic model of rat was reproduced by subcutaneouly injection of 60% CCl4 with a dose of 0.3 mL/100 g once every 4 days. Throughout the period, the rats were fed with ordinary food and 5% ethanol drinking water. Histological examination confirmed the development of cirrhosis at 60 days after initial ad ministration of CCl4.
In order to test ethanol tolerance of the normal and cirrhotic rats, laparotomy was performed in both normal ( n = 10 ) and cirrhotic rats ( n = 22 ) under intraperitoneal anesthesia with pentobarbital. After exposure of the hepatic hilum, portal vein was punctured with a 3-gauge needle and inject a dose of 0.05 mL/100 g of absolute ethanol. All rats in the normal group were alive but only 9 of 22 (40.9%) in the cirrhotic group survived 4 days after the injection.
Based on the technique and the results described above, embolization of the portal branches of left and central lobes (the embolized tissue accounted for 77% of the whole liver) was employed for 22 normal rats ( NE group ) with a dose of 0.05 mL/100 g of absolute ethanol. The portal branch of middle lobe (accounted for 36.5% of the liver) was embolized in 24 cirrhotic rats (ME group) with a dose of 0.03 mL/100 g. The same amount of normal saline was injected into portal vein in 10 normal rats (NC group) and 10 cirrhotic rats (MC group).
At day 1, 3, 7 and 14 after PVE, the following examinations were carried out: X-ray portography, the weight ratio of hepatic lobes, liver function tests (ALT, TBIL, ALP, ALB, A/G), liver histology, portal blood flow and portal pressure measured by MRF-1200 electromagnetic flowmetry ( Nikon, Japan).
PVE was undertaken under local anesthesia. A portal branch supplied the tumor bearing segment was punctured percutaneously under the guidance of Aloka SSD 650 or 1200 ultrasound system and 3.5 MHz linear puncture probe. In order to identify the precision of the puncture and whether or not a retrograde blood flow was present, portoechography was initially introduced with injection of 5 mL carb on dioxide (CO2). If the diffusion of CO2 was beyond the ipsilateral lobe of the liver, PVE was abandoned in case of aberrant embolization; otherwise PVE was performed with intraportal injection of 95% ethanol. The dose of ethanol ranged from 4mL to 10mL depending on the level the portal branch to be blocked.
From February 1993 to February 1993, portoechography was performed in 73 patients with HCC. But was quit in 12 cases due to CO2 diffusing into both lobes of the liver. Sixty-one patients (55 males and 6 females) with an average age of 52.5 years received PVE. Forty-nine patients had cirrhosis without jaundice, ascites or serious liver function had damage[6]. The tumors ranged from 3 cm to 12 cm in diameter, located at right lobe in 58 and left lobe in 15. All 61 patients underwent surgery 3-5 days after PVE including hepatectomy in 37 patients.
One dog in group B died of wound infection on day 5. Four of 7 dogs in group C died of respiratory arrest on the day of the injection.
Intrahepatic portography and dissection of the portal system showed the injected portal branches were patent at 30 min and 60 min after embolization. Thrombosis was developed on day 1. In group A, portal vein embolization occurred at one segment of the liver in six dogs and failure of embolization in one dog. In groups B and C, embolization occurred at both left and central lobes in most dogs. In one dog of group B, the orifice of the branch of the quadrate lobes was approximate to the branch of left lobe, and overembolization involving the quadrate lobes occurred. No thrombus was detected at the non-injected branches in any other dogs.
Macroscopically, the embolized lobes were swollen and congested but without any evidence of necrosis. Darked red thrombus had developed within the ethanol-injected portal branches on day 1 or 3, which were organized one week after PVE. Microscopically, focal inflammation of the intima was found in the affected portal branches. Small foci of coagulation necrosis prominently surrounding Glisson cap sule were detected at the embolized lobes and the normal structure of the lobule s was preserved. The necrotic area was less than 10% of the effected lobes. Tran sient elevation of white blood cell and alanine aminotransferase (ALT) occurred at day 1 but returned to the baseline values within one week. The level of total bilirubin, albumin and γ-globulin did not significantly change after PVE.
The survival rate after PVE was 95.8% in both NE and ME group. Postoperative portography demonstrated filling defects at injection sites and dissection of the portal system indicated the corresponding portal branches were embolized. The weight of the embolized hepatic lobes decreased gradually as the weight of non-embolized lobes increased with the time after PVE (Figure 1). On day 14, the weigh t of nonembolized lobes was significantly greater than the baseline value in both NE group (5.25 g ± 0.38 g vs 1.86 g ± 0.42 g, P < 0.01) and ME group (9.58 g ± 1.10 g vs 5.13 g ± 0.53 g, P < 0.01) and ratio of the nonembolized lobes to the whole liver significantly increased from 23% to 68.8% in NE group and from 63.5% to 86.8% in ME group.
Histological findings on day 1 or 2 were similar to that in the experiment of normal dogs. With respect to the ratio of total necrosis area to embolized area, ME group (30%-40%) was more severe than NE group (10%-20%). One week after PVE, organization or calcification of thrombi with partial recanalization as well as hyperplasia of fibrotic tissue in the embolized lobes was noted. Hepatocytes of the non-embolized lobes became hypertrophied and proliferative, which were more remarkable in ME group than in NE group.
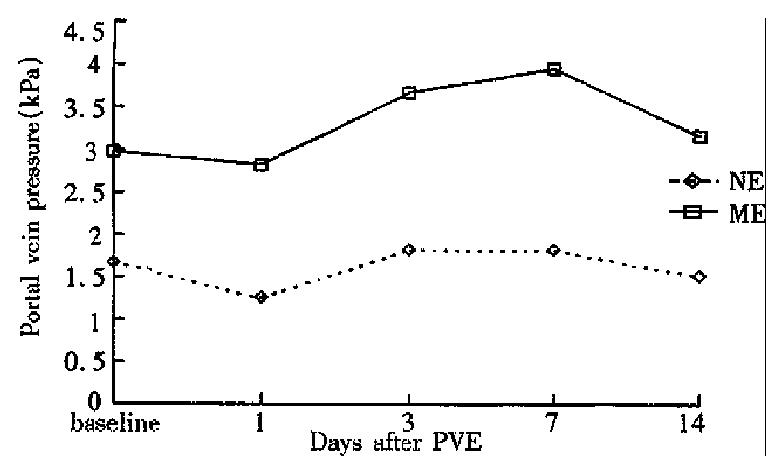
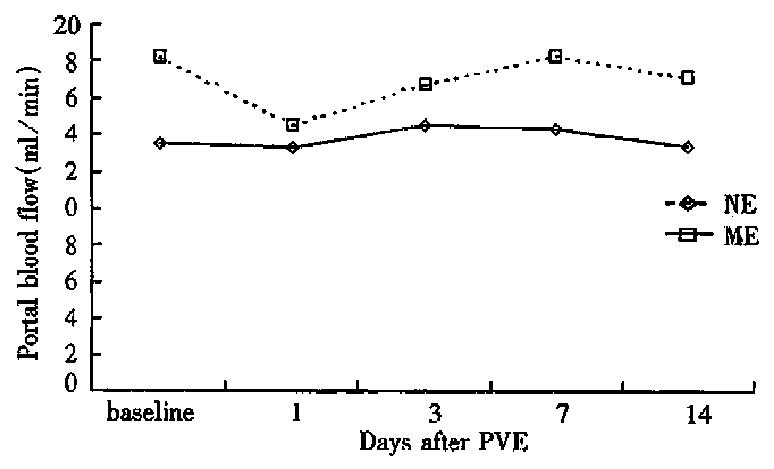
The hemodynamic study demonstrated that ME group had significantly higher portal blood flow and portal pressure than NE group before PVE. On day 1 after PVE, portal blood flow and portal pressure declined slightly in both groups, then increased to a limited degree and returned to the baseline values at week 1 after PVE (Figures 2 and 3).
The serum ALT, TBIL and ALP were elevated. After PVE, they began to fall on day 3 and returned to the pre-PVE level. The albumin and A/G ratio did not significantly change in both groups after PVE.
The punctures were confirmed to be proper by portoechography in all cases. By CO2 injection, a regional liver parenchyma was enhanced as high-echo pattern and developed a hyperechoic ring surrounding the tumors as our previous observation[7]. The echo within the tumors remained unchanged in 64 patients, but enhanced in 9 patients which represented portal blood stream within the tumors.
Enhancement of the hepatic parenchyma was localized to the area supplied by the injected branches or confined in the same lobe in 61 patients and subsequent PVE was performed. PVE was abandoned in 12 cases due to CO2 diffusing into both lobes of the liver.
Ultrasonography did not reveal any abnormality in the injected portal branches on the day of PVE. Forty-eight hours after PVE, embolization was found in 55/61 patients (90.2%), exhibiting substantial hypoechoes within the lumen of portal vein. Of 37 patients who underwent hepatectomy afterwards the specimen inspection confirmed the injected portal veins were occluded by thrombosis. Embolization failed to develop in six patients, who were the initial cases of PVE, due to inadequate amount of ethanol ( 4 mL-5 mL ) injected.
Forty-five patients (73.8%) complained of abdominal pain at the right upper quadrant, sweating, transient low-grade fever or decreased pulse rate during ethanol injection. Acute ischemic cholecystitis was encountered in one patient, for which the needle may be displaced accidentally during the injection, resulting in vascular occlusion of the gallbladder. Accidental embolization by reflux ethanol was not found in all patients and the liver function tests did not show significant change following PVE.
Comparing with transcatheter PVE, the advantages of PVE with fine needle are obvious: easy to achieve selective embolization, simple in manipulation, inexpensive and radiation free. However, at least two questions have to be answered which are prerequisite for conducting this procedure. The first question is about the safety of this procedure and the second is how to avoid a possible reflux of the ethanol to other portal branches. Furthermore, the feasibility of this technique should be convinced clinically.
Absolute ethanol is an effective embolizer in PVE. However, its toxic effect should not be neglected. In the present study, the dose of 0.25 mg/kg had little toxic effect but failed to induce complete embolization. While the dose of 1.0 mg/kg caused complete embolization but resulted in severe liver dysfunction and even respiratory arrest. Satisfactory embolization could be obtained at a dose of 0.5 mg/kg in normal dogs with mild toxicity to the liver parenchyma and only transient changes in the liver function. In transcatheter PVE with ethanol injection while the portal was occluded to blood flow, a rapid vascular obliteration and immediate embolization with entire necrosis at the affected region occurred[8]. On the contrary, since the portal vein was not occluded during ethanol injection in our technique, most of the ethanol might be diluted by the slow blood stream rather than flushed up to the liver parenchyma. The thrombosis was resulted from agglutinations, coagulation of plasma proteins and local pylephlebitis, and the thrombosis development was relatively slow. Thus, the damage to the liver tissue was relatively mild. Such a mechanism may be more favorable for the patients with HCC and underlying cirrhosis. The local overembolization in one dog in our study was probably due to an extension of the thrombus after the injection. This complication could be prevented by carefully selecting a point for puncture that is not too close to the confluence of the portal branches.
Since 80% of the patients with HCC are associated with cirrhosis, it is necessary to investigate the effect of PVE on cirrhotic liver for a better orientation on its clinical application. The present study indicated the cirrhotic rats were less tolerant to ethanol than the normal rats, so the dose of ethanol used for PVE should be strictly controlled. On the other hand, once PVE was undertaken with a tolerant dose, the changes in histology, liver function and portal hemodynamics in cirrhotic rats were not more severe and persistent than those of normal rats. It indicated that an uneventful postoperative course could also be achieved in cirrhotic rats with an appropriate dose of ethanol.
In both normal and cirrhotic liver, the weight of nonembolic lobe was increased with hypertrophy and proliferation in the nonembolized lobe in the current study. Increase in mitotic index, DNA synthesis and the number and function of mitochondrial in these lobes has been reported[9,10]. The hypertrophy may be contributed to the increase of nourishing factors carried by portal blood flow to the nonembolized lobes. This mechanism may play an important role in the surgery for cirrhotic patients as the improvement of functional reserve on the nonembolized lobe, hence the resectibility of HCC increased and the risk of postoperative liver failure reduced.
The features of portal circulation in the liver with HCC and cirrhosis were considered when our approach was planned for clinical use. Portal flow reflux may occur due to the circulatory disturbances within the tumor, portal branch compressed by huge mass, serious cirrhosis, portal hypertension and arteriovenous shunts. According to our previous portoechography and color Doppler study on patients with HCC, the incidence of portal vein reflux was about 10%-30%[11]. Since the injected portal branches were not be occluded during ethanol injection, accidental embolization could occur as the embolic agent might be carried to other branches by the reverse flow. In order to avoid the accidental embolization, it is important to obtain the information of portal hemodynamics individually prior to the procedure. Matsuda and Yabuuchi first reported a method of contrast-enhanced ultrasonography with arterial infusion of CO2 microbubbles for assessment of the nature of liver tumor[12]. Because the ultrasonic impedance of CO2 gas is much different from that of the liver and the gas can be rapidly washed out off the liver. It possesses excellent contrast effect without hepatic injury. This technique has been demonstrated as high sensitivity and specificity in clinical use[13,14]. Furthermore, the CO2 was directly injected into the destined portal branches, such portoechography could truly reveal the hemodynamics status of the portal branch[7]. In addition, it can be conveniently applied during the procedure of PVE. In the present study, reflux of portal stream was detected by portoechography in 12/73 patients and subsequent embolization was ceased for those patients. No accidental aberrant embolization occurred in the patients with CO2 confined at ipsilateral half of the liver, substantiating the usefulness of portoechography in our procedure.
The present study demonstrated that intraportal ethanol injection via fine needle was able to produce complete portal vein embolization with mild liver injury in both normal and cirrhotic liver in animal and patients with HCC. Portoechography was a mandatory approach for the prevention of aberrant embolization in patients with HCC.
Edited by Xie-Ning Wu
Proofread by Qi-Hong Miao
| 1. | Shimamura T, Nakajima Y, Une Y, Namieno T, Ogasawara K, Yamashita K, Haneda T, Nakanishi K, Kimura J, Matsushita M. Efficacy and safety of preoperative percutaneous transhepatic portal embolization with absolute ethanol: a clinical study. Surgery. 1997;121:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Nagino M, Nimura Y, Kamiya J, Kondo S, Uesaka K, Kin Y, Kutsuna Y, Hayakawa N, Yamamoto H. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677-681. [PubMed] |
| 3. | Kawasaki S, Makuuchi M, Kakazu T, Miyagawa S, Takayama T, Kosuge T, Sugihara K, Moriya Y. Resection for multiple metastatic liver tumors after portal embolization. Surgery. 1994;115:674-677. [PubMed] |
| 4. | Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803-808. [PubMed] |
| 5. | Fujio N, Sakai K, Kinoshita H, Hirohashi K, Kubo S, Iwasa R, Lee KC. Results of treatment of patients with hepatocellular carcinoma with severe cirrhosis of the liver. World J Surg. 1989;13:211-217; discussion 211-217;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Liang LJ. [Two-stage resection for advanced hepatocellular carcinoma--preliminary results of 13 cases]. Zhonghua Zhongliu Zazhi. 1992;14:449-451. [PubMed] |
| 7. | Lu MD, Liang LJ, Xie XY, Li DM, Li MD, Xie YY, Peng BG. Hepatic angio echography and its clinical application. Zhonghua Chaosheng Yingxiangxue Zazhi. 1993;2:154-156. |
| 8. | Ogasawara K, Uchino J, Une Y, Fujioka Y. Selective portal vein embolization with absolute ethanol induces hepatic hypertrophy and makes more extensive hepatectomy possible. Hepatology. 1996;23:338-345. [PubMed] [DOI] [Full Text] |
| 9. | Lee KC, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Harada H, Imamura H, Miyagawa S, Kawasaki S. Fate of the human liver after hemihepatic portal vein embolization: cell kinetic and morphometric study. Hepatology. 1997;26:1162-1170. [PubMed] |
| 11. | Lu MD, Xie YY, Liang LJ, Huang JF, Cao XH. Portal hemody-namics in hepatocellular carcinoma: observation by angio-echography and color Doppler. Zhonghuo Chaosheng Yixue Zazhi. 1994;10:24-27. |
| 12. | Matsuda Y, Yabuuchi I. Hepatic tumors: US contrast enhancement with CO2 microbubbles. Radiology. 1986;161:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Kudo M, Tomita S, Tochio H, Kashida H, Hirasa M, Todo A. Hepatic focal nodular hyperplasia: specific findings at dynamic contrast-enhanced US with carbon dioxide microbubbles. Radiology. 1991;179:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Takasaki K, Saito A, Nakagawa M. Significance of angioechography for diagnosis of small intrahepatic metastasis. Kanzuo. 1988;29:917-920. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |