Published online Dec 15, 1999. doi: 10.3748/wjg.v5.i6.465
Revised: September 16, 1999
Accepted: October 10, 1999
Published online: December 15, 1999
AIM: To determine whether incorporation of the pH-dependent ba cterial toxin listeriolysin O (LLO) into the DNA carrier system could increase the endosomal escape of internalized DNA and result gene expression.
METHODS: A multi-component delivery system was prepared consist ing of asialoglycoprotein (ASG), poly L-lysine (PL), and LLO. Two marker genes, luciferase and β-galactosidase in plasmids were complexed and administered in vitro to Huh7[ASG receptor (+)] and SK Hep1 [ASG rece ptor(-)] cells. Purity, hemolytic activity, gene expression, specificity, and toxicity were evaluated.
RESULTS: An LLO-containing conjugate retained cell-targeting specificity and membranolytic activity. In ASG receptor (+) cells, luciferas e gene expression was enhanced by more than 7-fold over that of conjugates with out the incorporation of listeriolysin O. No significant expression occurred in ASG receptor (-) cells. Enhancement of β-galactosidase gene expression was less, but still significantly increased over controls. There was no detectable toxicity at concentrations shown to be effective in transfection studies.
CONCLUSIONS: ASOR-PL can be coupled to LLO using disulfide bonds, and successfully target and increase the gene expression of foreign DNA.
- Citation: Walton CM, Wu CH, Wu GY. A DNA delivery system containing listeriolysin O results in enhanced hepatocyte-directed gene expression. World J Gastroenterol 1999; 5(6): 465-469
- URL: https://www.wjgnet.com/1007-9327/full/v5/i6/465.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i6.465
We have previously demonstrated targeted delivery of DNA to the liver via the recognition of asialoglycoprotein-polylysine (PL) containing conjugates by the hepatic asialoglycoprotein (ASG) receptors[1,2]. Binding of the ASG-PL-DNA complexes to the ASG receptors resulted in internalization of the ligand-rece ptor complex into the cells via receptor-mediated endocytosis. However, a probl em with using receptor-mediated endocytosis as a gene delivery system is that once the specific ligand-DNA complex is internalized, it must escape the endosom e before delivery to the lysosome in order to avoid intracellular degradation. The endocytosis pathway is somewhat “leaky”, which allows for the escape of some DNA without additional membrane disruption. However, most of the delivered genetic material remains trapped in the endosome and is degraded by lysosomal proteases. This may account for low transfection efficiency and transient expression[3].
To address this problem we sought to use the natural properties of a bacterial toxin to enable targeted DNA to escape from endosomal vesicles. Listeriolysin O (LLO) is a pH dependent, thiol-activated, membranolytic protein secreted by the bacteria Listeria monocytogenes. LLO is necessary for the pathogenicity of L. monocytogenes. Ingested bacteria are taken up by host cells, primarily by macrophages, into phagolysosomes. However, the organism secretes LLO in the pha golysosome, where upon acidification, the LLO undergoes a conformational change, resulting in rupture of the vesicle. Escape of the bacteria from the vesicle occurs before contact with lysosomal enzymes allowing for further replication in the cytosol[4,5].
In the following report, we demonstrate that the incorporation of LLO into the ASG-PL carrier system results in increased gene expression while retaining cell type specificity.
ASOR and polylysine were chemically conjugated using a water soluble carbodiimide as described previously[6,7]. In brief, ASOR, prepared by desialylation of orosomucoid from pooled human serum[6], was mixed with PL, MW=38500 (Sigma Chemical Co.), in a 1:1 weight ratio. The reactants were coupled by addition of 1-ethyl-3-(3-dimethylaminopropyl )-carbodiimide ( Pierce Chem ical Co. ) and purified by cation exchange chromatography. LLO was purified using a CH2 spiral concentrator (Amicon) and DEAE Sephacel column (Pharmacia Fine C hemicals). The cleavable cross linker N-succinimidyl 3-(2-pyridyldithio) propionate, SPDP, (Pierce Chemical Co.) was added to both ASOR-PL and LLO proteins according to the Pierce protocol. One milligram of both proteins was incubated with 25mM SPDP in dimethylsulfoxide for 30 minutes at room temperature. Free SPDP was se parated from linked by application to a PD-10 desalting column (Pharmacia Fine Chemicals) and elution with water. The concentration of SPDP linked to the proteins was determined by measuring the release of 2-thione after reduction with 100 mM DTT and reading the absorbance at 343 nm. The LLO-SPDP was activated f or coupling by reduction with 12mg DTT in 100 mM NaCl, 100 mM Na acetate pH 4.5. Free DTT was removed by application to a PD-10 desalting column and elution with water.
The reporter genes used in these experiments were either CMV luciferase (CMV luc) or β-galactosidase (β-gal) plasmid DNA. The ratio of conjugate needed to bind a specific amount of DNA was determined by a dding increasing amount of ASOR-PL-SPDP to 1 mg of CMV luc or β-gal plasmid DNA in 0.15 M saline. These samples were run on 1% a garose gels, and the point of DNA retardation was visualized by staining with ethidium bromide and observation with UV. This ratio was used in subsequent experiments. One milligram of the SPDP linked ASOR-PL and CMV luc or β-gal plasmid DNA in the proper proportion were incubated for 30min at room temperature in 0.15M saline. The ASOR-PL-SPDP-DNA complex was added to DTT reduced LLO-SPDP in a 2:1 molar ratio. The conjugate was incubated overnight at 4 °C and filtered through a 0.2 μm Nalgene syringe filter.
The conjugate was characterized by Western blotting with polyclonal antibodies to LLO (Immune Response Corporation). One milligram of LLO and the final conjugat e either with or without DTT (100 mM) reduction were run on a 7.5% SDS-PAGE gel. The proteins were transferred to a nylon membrane (Amersham), quenched for one hour in 5% dry milk dissolved in 10 mM Tris, 150 mM NaCl and 0.5% Tween-20, and probed in the same buffer with a polyclonal antibody to LLO. Detecti on of the antigen-antibody complex was determined by exposure to anti-rabbit IgG horseradish peroxidase (Sigma) and developed with 3’,3’diaminobenzidine and hydrogen peroxide (Sigma)[9].
To determine if the DNA remained bound conjugates under experimental conditions, agarose gels were run. One microgram of DNA bound to conjugate was run in an 1% agarose gel and visualized with ethidium bromide and UV.
Hemolytic activity was determined by the adding the conjugate to one milliliter of PBS, pH 5.5, and 5 mM DTT plus 6 × 108 human red blood cells. Samples were incubated for 30 minutes at 37 °C and quantified by absorbance at 541 nm on a spect rophotometer. One hemolytic unit (HU) is the amount of LLO needed to release hem oglobin from 50% of the red blood cells[10].
Conjugates containing 1 μg of CMV luc or β-gal DNA were added to Huh7 (ASG receptor positive) or SK Hep1 (ASG receptor negative) cells in 1 mL of DMEM containing 2 mM CaCl2 and incubated for 4 h at 37 °C. Then FBS was added to a final concentration of 10% and cells were further incubated for 48 h. ASOR-PL-DNA, ASOR-PL-DNA plus free LLO, and ASOR-PL-LLO-DNA plus a 200-fold excess ASOR were used as controls. Gene expression was measured by luciferase detection using the luciferin substrate (Promega), and detection of activity using a luminometer. Luciferase expression results were standardized by measuring protein concentrations according to the Bradford assay[9].
Cells incubated with β-gal were washed with PBS pH 7.4, fixed with 4% paraformaldehyde, and stained with X-gal (20 μg/L in dimethylformamide) for 30 min at 37 °C. Cells were observed with light microscopy and positive (blue stained) cells were counted.
Huh7 and SK Hep1 cells were incubated with ASOR-PL-DNA, free LLO, ASOR-PL-LL O-DNA or ASOR-PL-LLO-DNA in the presence of a 200-fold excess of ASOR in Du lbecco’s minimal essential media (DMEM) + 2 mM CaCl2 for 4 h at 37 °C. Fetal bovine serum (FBS) in a final concentration of 10% was added, and the cells w ere further incubated for 24 and 48 h at 37 °C. Cell viability was determined by trypan blue exclusion[11].
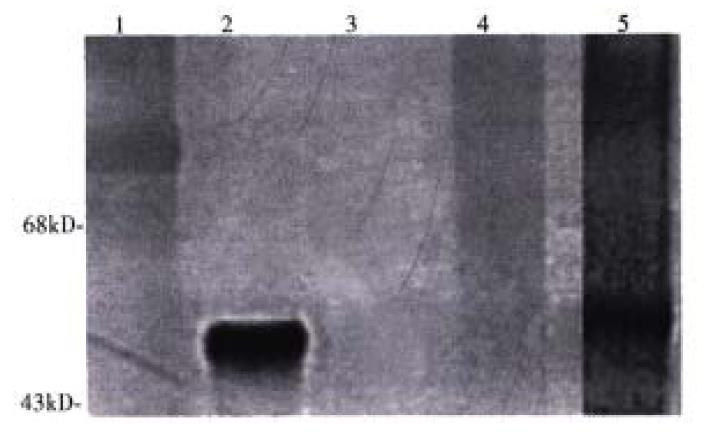
The molar ratio of SPDP linked to ASOR-PL and LLO, determined by the release of 2-thione after reduction, was found to be 1:1. The ratio of ASOR-PL-SPD P needed to retard migration of DNA in agarose gels was 114:1 moles. ASOR- PL-LLO-DNA complexes were analyzed on a Western blot with a polyclonal antibod y to LLO. As shown in Figure 1, purified LLO migrated at a position expected for Mr 58000, lane 2. Molecular weight markers are shown in lane 1. ASO R-PL alone, as expected, did not bind to the antibody indicating that the ASOR-PL conjugate itself was not capable of non-specific binding with the anti-LLO antibody, lane 3. However, after coupling to LLO, the conjugate did react with anti-LLO antib ody, and this conjugate was found not to migrate into the gel, lane 4. No contaminating bands were visualized. Chemical reduction of the complex with DTT resulted in a free band migrating at the position of LLO, lane 5. An agarose gel of the conjugate revealed no migrating bands, indicating that DNA remained bound (data not shown).
The results of hemolytic assays are shown in Table 1. LLO alone, as expected, was highly hemolytic at pH 5.5, but only minimally active at pH 7.4 when concentrations were low. However, at high concentrations, greater that 0.5 μg/mL, the pH had little effect. Hemolytic assays demonstrated that hemolytic activity of the conjugate was concentration dependent. Similar to LLO alone, the highest activity occurred at pH 5.5. At pH 7.4, the conjugate did not cause appreciable hemolysis. This suggested the conjugation procedure does not appreciably alter the hemolytic characteristics of the LLO, and that at phys iological pH, the conjugate has no active LLO.
| LLO (μg) | LLO | ASOR-PL-LLO-DNA | ||
| pH 5.5 | pH 7.4 | pH 5.5 | pH 7.4 | |
| 0.005 | 1.000 | 0.024 | 0 | 0 |
| 0.010 | 1.380 | 0.051 | 0 | 0 |
| 0.050 | 1.680 | 0.074 | 0.010 | 0 |
| 0.100 | 1.900 | 1.280 | 0.021 | 0 |
| 0.500 | 1.900 | 1.730 | 0.349 | 0 |
| 1.000 | 2.100 | 1.900 | 0.947 | 0.013 |
| 2.000 | 2.100 | 2.100 | 1.900 | 0.020 |
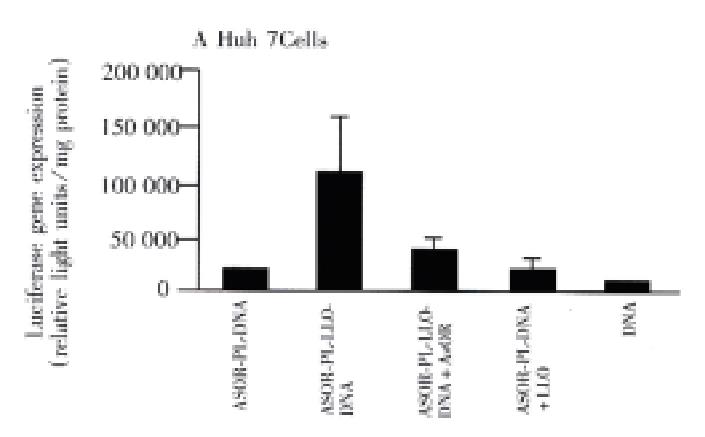
Figure 2, panel A, shows that prototype ASOR-PL-DNA complexes introduced into Huh7 [ASG receptor positive]cells produced approximately 5000 light units, lane 1. However, compared to the prototype, ASOR-PL-LLO-DNA complexes produced luciferase activity 7 times higher, lane 2. This enhancement was decreased by 60% with the addition of free ASOR to compete with the complex for ASG receptors, lane 3. The addition of free (not conjugated) LLO to ASOR-PL complexes in exactly the same molar concentration as provided by the ASOR-PL-LLO conjugate did no t enhance luciferase gene expression, lane 4. This indicates that the observed e nhancement of transfection could not be due to the effects of any free LLO. DNA alone had no significant gene expression, lane 5. In SK Hep1[ASG receptor negative] cells there was no significant gene expression with prototype, lane 1, or ASOR-PL-LLO-DNA complexes, lane 2. Of course, controls consisting of addition of LLO to prototype ASOR-PL complexes, and DNA alone in this cell line alone h ad no detectable levels of luciferase expression, lanes 3 and 4, respectively.
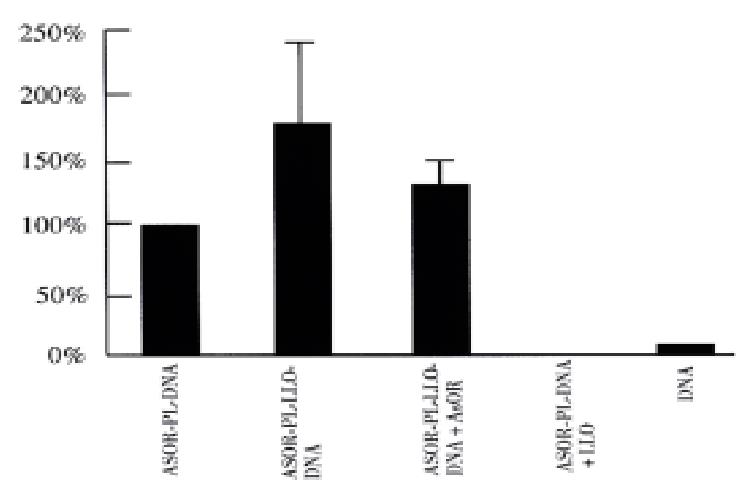
Figure 3 shows the results of studies of Huh7 cells transfected with a gene for β-galactosidase. The ASOR-PL-LLO-DNA complex containing β-gal DNA increased gene expression 185% over prototype ASOR-PL complexes (complex lacking LLO), lane 2. The enhancement was inhibited by 30% with addition of a 200-fold excess ASOR, lane 3. ASOR-PL-DNA plus free LLO, and DNA alone had no significant gene expression, lanes 4 and 5, respectively.
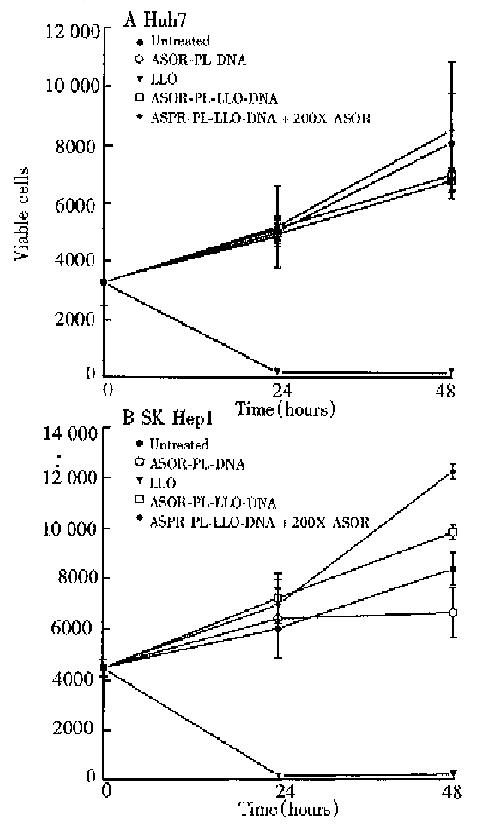
In order to determine whether the LLO-containing conjugate was toxic, cell viability studies were performed and the results are shown in Figure 4. Huh7 and SK Hep1 cells, panels A and B respectively, treated with ASOR-PL-DNA, ASOR-PL-L LO-DNA with or without an excess of ASOR proliferated at the same rate as control (untreated) cells. However, LLO alone in the same concentration as present in the ASOR-PL-LLO-DNA repidly decreased viable cell numbers. Thus, the concent ration of complexes used for expression studies were found not to be toxic to either Huh7 or SK Hep1 cells.
Many approaches have been developed to solve the problem of low transfection efficiency and transient expression of gene delivery systems. Partial hepatectomy, chloroquine administration, and viruses or viral peptides have shown to be usedful in increasing in the duration of expression[6,12-16]. The current report is the first demonstration that the natural escape strategies of the bacterial toxin LLO can be useful as a potential component of a targetable DNA delivery system to increase efficiency[4,5].
LLO is a thiol-containing protein[5]. Experiments on direct bonding to ASOR-PL-SPDP resulted in low efficiency (data not shown). In subsequent experiments LLO was also treated with SPDP to increased the number of potential cross linking residues. Subsequent reduction with DTT resulted in successful conjugation.
The conjugated LLO had lower hemolytic activity compared to equal amounts of free LLO. This was in spite of the fact that DTT is added to samples to activate the thiol groups. This reduction would also cleave coupled LLO from the conjugate. The lower molar activity of the released LLO may have been due to the addition of SPDP, or to interference from ASOR or DNA that was independent of thiol reduction. Nevertheless, the hemolytic activity of the conjugate was pH dependent as seen with native LLO. This is important as one of the theoretical advantages for the use of LLO was the lack of activity at physiological pH, and restored activity in the acidic environment of the endosome. Toxicity due to extracellular activity would be minimized.
In summary, the above experiments show that ASOR-PL can be coupled to LLO using disulfide bonds, and successfully target and increase the gene expression of foreign DNA. The increase in expression was blocked with the addition of a large molar excess of ASOR, and was present only in ASG receptor positive cells, indica ting retention of hepatocyte specificity.
The technical assistance of Ying Zhang, and the secretarial assistance of Martha Schwartz are gratefully acknowledged. This work was supported in part by a grant from the National Institutes of Health, DK-42182 (GYW), the Immune Response Corporation (GHW), and the Herman Lopata Chair for Hepatitis Research.
Edited by MA Jing-Yun Proofread by MIAO Qi-Hong
| 1. | Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987;262:4429-4432. [PubMed] |
| 2. | Wu GY, Wu CH. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988;263:14621-14624. [PubMed] |
| 3. | Cotten M, Baker A, Saltik M, Lehrmann H, Panzenbock B, Chiocca S. Receptor mediated gene delivery. In: Blum HE, ed. Molecular diagnosis and gene therapy. Boston MA: Kluwer Academic Publishers. 1996;80-91. |
| 4. | Geoffroy C, Gaillard JL, Alouf JE, Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987;55:1641-1646. [PubMed] |
| 5. | Vazquez-Boland JA, Dominguez L, Rodriguez-Ferri EF, Suarez G. Purification and characterization of two Listeria ivanovii cytolysins, a sphingomyelinase C and a thiol-activated toxin (ivanolysin O). Infect Immun. 1989;57:3928-3935. [PubMed] |
| 6. | Wu GY, Zhan P, Sze LL, Rosenberg AR, Wu CH. Incorporation of adenovirus into a ligand-based DNA carrier system results in retention of original receptor specificity and enhances targeted gene expression. J Biol Chem. 1994;269:11542-11546. [PubMed] |
| 7. | McKee TD, DeRome ME, Wu GY, Findeis MA. Preparation of asialoorosomucoid-polylysine conjugates. Bioconjug Chem. 1994;5:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Walton CM, Wu CH, Wu GY. A method for purification of listeriolysin O from a hypersecretor strain of Listeria monocytogenes. Protein Expr Purif. 1999;15:243-245. [PubMed] |
| 9. | Harlowe E, Lane D (eds). Antibodies, a laboratory manual. Cold Spring Harbor Laboratories. 1988;. |
| 10. | Cossart P, Vicente MF, Mengaud J, Baquero F, Perez-Diaz JC, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629-3636. [PubMed] |
| 11. | Garvey JS, Cremer NE, Sussendorf DN (eds). Methods in immunology. 3rd ed. Reading MA: WA Benjamin, Inc 1997; 449. |
| 12. | Wu CH, Wilson JM, Wu GY. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J Biol Chem. 1989;264:16985-16987. [PubMed] |
| 13. | Chowdhury NR, Hays RM, Bommineni VR, Franki N, Chowdhury JR, Wu CH, Wu GY. Microtubular disruption prolongs the expression of human bilirubin-uridinediphosphoglucuronate-glucuronosyltransferase-1 gene transferred into Gunn rat livers. J Biol Chem. 1996;271:2341-2346. [PubMed] |
| 14. | Feng M, Jackson WH, Goldman CK, Rancourt C, Wang M, Dusing SK, Siegal G, Curiel DT. Stable in vivo gene transduction via a novel adenoviral/retroviral chimeric vector. Nat Biotechnol. 1997;15:866-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 95] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Schlegel R, Wade M. Biologically active peptides of the vesicular stomatitis virus glycoprotein. J Virol. 1985;53:319-323. [PubMed] |
| 16. | Wagner E, Plank C, Zatloukal K, Cotten M, Birnstiel ML. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin-polylysine-DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc Natl Acad Sci USA. 1992;89:7934-7938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 444] [Article Influence: 13.5] [Reference Citation Analysis (0)] |