Published online Aug 15, 1999. doi: 10.3748/wjg.v5.i4.316
Revised: March 3, 1999
Accepted: April 19, 1999
Published online: August 15, 1999
AIM: To study the telomerase expression in gastric carcinoma and its clinical implications.
METHODS: Telomerase activity was examined in gastric cancer an d corresponding normal tissues using a modified TRAP (telomeric repeat amplification protocol) assay (TRAP-eze) in tissue samples from 94 gastric carcinomas an d 58 normal tissues, 12 gastric adenomas and 9 gastric ulcer lesions.
RESULTS: Telomerase activity was present in 81 of the 94 (86.2%) gastric cancer tissues, whereas no telomerase activity was detected in any normal tissues. The incidence of telomerase activity in gastric cancer tissues was unrelated to the tumor diameter, histological grade, tumor invasion in depth, lymph node metastasis and TNM stage.
CONCLUSION: Telomerase plays an important role in carcinogenes is and progression of gastric cancer, and it is suggested to be a useful tumor marker.
- Citation: Zhan WH, Ma JP, Peng JS, Gao JS, Cai SR, Wang JP, Zheng ZQ, Wang L. Telomerase activity in gastric cancer and its clinical implications. World J Gastroenterol 1999; 5(4): 316-319
- URL: https://www.wjgnet.com/1007-9327/full/v5/i4/316.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i4.316
The development of carcinoma results from multiple independent genetic changes t hat activate proto-oncogenes or inactivate the action tumor suppressor genes. Although several authors have reported findings in gastric cancer that support t his concept[1], the molecular mechanism of gastric carcinogenesis has not been fully explored. Recent studies showed that telomerase activity expression was closely correlated with the occurrence and development of human malignant tumors. Telomerase activity expression was detected in most of human tumors[2,3].
Telomerase is a ribonuceloprotein complex that adds telomeric repeats onto chromosomal ends using an intrinsic RNA as a template, thus extending telomeric DNA[4,5]. Telomeres are special structures containing unique repeats and associated proteins at the ends of eukaryotic chromosomes, which consist of short tandem DNA repeat sequences and telomere-binding proteins. Human telomeres repetitive sequence is TTAGGG/AATCCC. Telomeres are thought to be important for protecting the ends of the chromosomes from fusion, degradation and random breakage, and for maintaining the stability of the chromosomes. Telomeres apply for the important structure fundament for solving the so-called "end replication problem". According to the so-called "end replication problem", the cellular chromosomes lose 50-200 bp of telomeric sequences during each cell division, leading to a limited replicative capacity and eventually resulting in cellular senescence. Therefore, telomere is considered to play a role of "mitotic clock"[6,7]. In germline cells, telomere length maintained stable because of telomerase enzyme, which resynthesizes telomeric DNA sequences at chromosome ends, in contrast, telomere length is kept balanced because of telomerase reactivity in the malignant cells.
During the past 20 years, the mortality rates of gastric cancer have been rising constantly in our country, and the mortality rate of gastric cancer ranks first in the analyses of variation trends and short-term detection of Chinese malign ant tumor mortality[8]. Therefore, it is urgent to put emphasis on the study of the experimental and clinical studies. The study of telomerase expression in primary gastric cancer could help us better understand the carcinogenesis of gastric cancer at molecular level, and search for a new gastric tumor marker, which are of benefit for development of drugs or methods to inhibit telomerase activity[9].
The non-isotopic TRAP assay was used in this study to detect telomerase activity expression and clinical pathological data in gastric carcinomas.
Surgically resected tissue samples from 94 gastric carcinomas, 58 normal gastric mucosa tissues, 12 gastric adenomas and 9 gastric ulcer lesions were taken from operations performed during the period of February 1997 to Septemper 1998. The gastric cancer samples were collected from 50 males and 44 females aged from 30 to 82 years (mean age 63). The mean diameter of tumors is 4.6 cm (ranging from 2 cm to 12 cm), and according to the depth of invasion, there we e 2 of T1, 14 of T2, 38 of T3 and 40 of T4. Two were of stage I, 23 of stage II, 42 of stage III, and 27 of stage IV. Tumor sizes were measured immediately after gastrectomy. All samples were obtained within 1 h after surgical removal. Specimens were snap frozen and stored in liquid nitrogen until use. The histopathological factors were evaluated by our pathologists and operators according to the international established criteria.
Frozen samples of 50-100 mg, including gastric cancer, normal gastric mucosa, gastric adenoma and metaplastic lesions, were washed twice with ice wash buffer, all of which was then carefully removed. The samples were kept in liquid nitrogen and ground with a pestle until a smooth consistency was achieved, and then homogenized until a uniform consistency was reached and mixed with 200 μL × CHAP Lysis Buffer. After 30 min incubation on ice, the lysates were centrifuged at 16000 rpm for 30 min at 4 °C. The 160 μL supernatant was placed into two fresh tubes, and the protein concentration was determined. The remainin g extract was aliquoted and quickly frozen and stored at -80 °C.
All operational procedures were finished on the super-clean desk in ice water, and all reagents were thawed on ice. The amount of reagent required in each TRAP assay is 50.0 μL containing: 10 × TRAP buffer 5.0 μL, 50 × dNTP-s mixture 1.0 μL, TS primer 1.0 μL, CX primer [5′(CCCTTA)3-CCCTAA 3′]1.0 μL, Taq polymerase (5 U) 0.4 μL (2 U), ddH2O3 9.6 μL and tissue extract 2.0 μL, which was incubated for 30 min at 30 °C. The reaction mixture was then subjected to 31 PCR cycles at 94 °C for 45 sec, 50 °C for 45 sec and 72 °C for 90 sec.
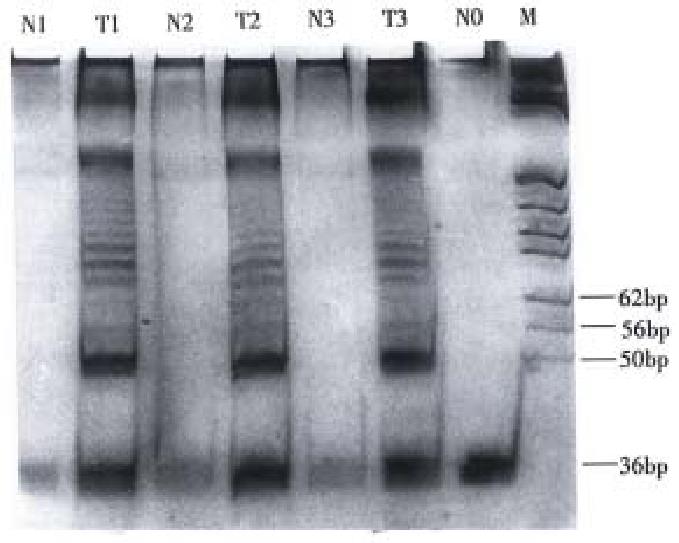
The PCR products were analyzed by electrophoresis in a 12.5% polyacrylamide nondenaturating gel for 1 h at 100 volts for a 10-12 cm vertical gel, or until the bromophenol blue just run off the gel. After electrophoresis, the gel was stained with silver nitrate solution. The positive enzyme activity was confirmed when the telomerase specific, 6 bp DNA ladder, was observed.
Statistical analysis was made with SAS. Telomerase positive ratio between groups was tested for significance with χ2 test and Fisher exact test.
The expression of telomerase in gastric carcinoma (Table 1).
| Groups | Telomerase activity | Positive rate(%) | |
| Positive | Negative | ||
| Gastric cancer (n = 94) | 81 | 13 | 86.2 |
| Normal tissue (n = 58) | 0 | 58 | 0 |
| Gastric ulcer (n = 9) | 1 | 8 | 11.1 |
| Gastric adenoma (n = 12) | 1 | 11 | 8.38 |
Telomerase activity was detected in 81 (86.2%) of 94 gastric carcinomas examined, 1 of 12 gastric adenoma and 1 of 9 gastric ulcer lesions. However, no telomerase activity (P < 0.001) was detected in all the 58 normal tissues from the same patients (Figure 1).
The relationship between telomerase activity and clinical pathological parameters in gastric carcinoma.
The incidence of telomerase activity in gastric carcinoma tissues was not correlated to tumor diameter, histological grade, tumor invasion in depth, lymph node metastasis and TNM stage (P > 0.05, Table 2).
| Groups | Telomerase activity | χ2 | P | |
| Positive(n = 81) | Negative(n = 13) | |||
| Tumor size (cm) | ||||
| < 5 (n = 42) | 35 | 7 | ||
| 5.8 (n = 32) | 29 | 3 | 0.839 | 0.657 |
| > 8 (n = 20) | 17 | 3 | ||
| Histological grade | ||||
| Well differentiated (n = 28) | 23 | 5 | ||
| Moderately and well differentiated(n = 18) | 16 | 2 | ||
| Poorly differentiated (n = 48) | 42 | 6 | 0.564 | 0.754 |
| Invasion depth | 1 | 1 | ||
| T1 (n = 2) | 1 | 1 | ||
| T2 (n = 14) | 12 | 2 | ||
| T3 (n = 38) | 33 | 5 | ||
| T4 (n = 40) | 35 | 5 | 0.392 | 0.531 |
| Lymph node metastasis | ||||
| < 3 cm (n = 38) | 30 | 8 | ||
| > 3 cm (n = 56) | 51 | 5 | 2.792 | 0.095 |
| TNM stage | ||||
| I (n = 2) | 1 | 1 | ||
| II (n = 23) | 20 | 3 | ||
| III (n = 42) | 37 | 5 | ||
| IV (n = 27) | 23 | 4 | 0.135 | 0.714 |
Telomeres, the distal ends of human chromosomes composed of tandem repeats of the sequence TTAGGG, are believed to play an essential role in maintaining the stability and protection of chromosomes. Normal human somatic cells lose 50-100 bp of terminal telomeric DNA with each round of replication. Telomerase is a ribonucleoprotein containing an RNA template that synthesizes telomeric DNA. Cells with the activated enzyme appear to escape from progressive telomeric shortening and acquire an indefinite growth potential[10]. Telomerase activity has been demonstrated in multiple tumor lines and is absent in corresponding normal tissues. In our study, telomerase activity was positive in 81 (86.2%) of 94 gastric carcinoma tissues, whereas it was negative in normal tissues. According to a recent review, 758 (85%) of 895 malignant tumors, but none of 70 normal somatic tissues, expressed telomerase activity[11]. These strong associations of telomerase activity with malignant tumors is good evidence that telomerase can be a n important marker for diagnosing cancer.
In our study, telomerase activity was also detected in 1 of 2 early gastric cancers, and 1 of 12 gastric adenomas and 9 gastric ulcer lesions, respectively. The results from Maruyama et al[12] are similar to those of the present study in which telomerase activity was detected in 10 (45%) of 22 adenomas and 2 (15%) of 12 metaplastic lesions. Tahara et al[14,15] demonstrated telomerase activity in 10 tubular adenoma samples. These results support the hypothe sis that telomerase activation is an early event in the malignant process. According to the previous report, telomerase activity expression could take place in the early stage of gastric cancer even in the precancerou s lesions, which implicated the importance of the early diagnosis of gastric cancer.
It is interesting that about 15% of malignant tumors expressed no telomerase activity, and in our study, no telomerase activity has been detected in about 14% gastric cancer samples. It is unclear what mechanism is responsible for this phen omenon. Long-term chromosome stability in humans depends on the addition of telomeric repeats, and telomerase is essential for telomere length maintenance. How ever, in a survey of immortal cell lines, Kim et al[16] found that approximately 10% of tumors maintained long stretches of terminal repeats without detectable telomerase activity. Bryan et al[17] also found that 40% of the immortal lines showed no telomerase activity, yet these cell lines had very long and heterogeneous telomeres of up to 50 kb. Recombination has been proposed as a possible elongation mechanism in these cases, however, direct evidence is still lacking[18]. In a recent research, Smith et al[19] found a second enzyme, tankyrase, which may enable telomerase to do its work. These evidence suggests that tankyrase can control the action of telomerase by removing TRF 1, a telomere-specific DNA binding protein that otherwise blocks telomerase′ s access to the chromosome ends.
We found no significant correlation between levels of telomerase activity and other clinicopathological variables, including tumor diameter, histological grade, tumor invasion in depth, lymph node metastasis and TNM stage. This is in contrast to the results of Hiyama et al[14], who reported that telomerase activity was present mostly in large tumors or those of advanced stage, including metastastic tumors. Furthermore, the survival rate of tumor patients with detectable telomerase activity was significantly lower than that of patients without telomerase activity. However, our results are in agreement with the studies by Ahn et al[15]. These contradictory results need further investigations.
Edited by MA Jing-Yun
| 2. | Rhyu MS. Telomeres, telomerase, and immortality. J Natl Cancer Inst. 1995;87:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 269] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Shay JW, Gazdar AF. Telomerase in the early detection of cancer. J Clin Pathol. 1997;50:106-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA. 1998;95:90-92. [PubMed] |
| 5. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [PubMed] |
| 6. | Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2511] [Cited by in RCA: 2637] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 8. | Li LD, Lu FZ, Zhang SW. Trend of change in mortality from malignant neoplasms over the past 20 years and predictive analysis. Chin J Oncol. 1997;19:3-9. |
| 9. | Morin GB. Is telomerase a universal cancer target? J Natl Cancer Inst. 1995;87:859-861. [PubMed] |
| 10. | Blasco MA, Rizen M, Greider CW, Hanahan D. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat Genet. 1996;12:200-204. [PubMed] |
| 11. | Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2004] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 12. | Maruyama Y, Hanai H, Fujita M, Kaneko E. Telomere length and telomerase activity in carcinogenesis of the stomach. Jpn J Clin Oncol. 1997;27:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Tahara H, Kuniyasu H, Yokozaki H, Yasui W, Shay JW, Ide T, Tahara E. Telomerase activity in preneoplastic and neoplastic gastric and colorectal lesions. Clin Cancer Res. 1995;1:1245-1251. [PubMed] |
| 14. | Hiyama E, Yokoyama T, Tatsumoto N, Hiyama K, Imamura Y, Murakami Y, Kodama T, Piatyszek MA, Shay JW, Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995;55:3258-3262. [PubMed] |
| 15. | Ahn MJ, Noh YH, Lee YS, Lee JH, Chung TJ, Kim IS, Choi IY, Kim SH, Lee JS, Lee KH. Telomerase activity and its clinicopathological significance in gastric cancer. Eur J Cancer. 1997;33:1309-1313. [PubMed] |
| 16. | Kim NW. Clinical implications of telomerase in cancer. Eur J Cancer. 1997;33:781-786. [PubMed] |
| 17. | Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240-4248. [PubMed] |
| 18. | Biessmann H, Mason JM. Telomere maintenance without telomerase. Chromosoma. 1997;106:63-69. [PubMed] |