Published online Jun 15, 1999. doi: 10.3748/wjg.v5.i3.231
Revised: February 18, 1999
Accepted: March 6, 1999
Published online: June 15, 1999
AIM: To study the effect of leukocyte-endothelium interaction (LEI) on the flow and distribution of leukocytes in microcirculation under physiological condition.
METHODS: A microcirculation image multiple parameter computer analysis system (MIMPCAS) was used to study the flow and distribution of leukocytes in mesentery microcirculation of rats in vivo.
RESULTS: The difference of visible leukocyte flux (VLF) was as high as 131 times in the arterioles and venules with similar diameter and blood velocity. The visible leukocytes rolled along the blood vessel wall as a “jerky” movement. The frequency distribution of the visible leukocyte velocity (VLV) showed a “two-peak” curve. The low peak value was on 10 μm/s-15 μm/s while the high peak fell between 25 μm/s-30 μm/s. With the increase of diameter of venules, VLF increased while the VLV remained at the same level. With the increase of RBC velocity, VLV trends to elevate and VLF to fall down.
CONCLUSION: The results herein might provide a basic theory for the study on the mechanism of LEI under physiological condition and novel methods for the prevention and treatment of high LEI in many pathological processes.
- Citation: Jiang Y, Liu AH, Zhao KS. Studies on the flow and distribution of leukocytes in mesentery microcirculation of rats. World J Gastroenterol 1999; 5(3): 231-234
- URL: https://www.wjgnet.com/1007-9327/full/v5/i3/231.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i3.231
Leukocyte-endothelium interaction (LEI) exists in many pathophysiological processes, such as inflammation, burns, tumor and shock[1,2]. In the recent two decades, quantitative studies on the interaction of leukocyte-endothelium have been carried out and the change of the flow and distribution of leukocytes is the basis for the abnormal increase of LEI[3-5]. High level LEI would b ring about the blockage of blood vessels and decrease of blood perfusion[6,7].Therefore, it is important to study the flow and distribution of leukocytes in microcirculation. We used a microcirculation image multiple parameter computer analysis system (MIMPCAS)[4] to study the characteristic of the flow and distribution of leukocytes in mesentery microcirculation of rats, and analyzed its influencing factors.
Five Sprague-Dawley rats were anesthetized with a mixture of 133 g/L urethane and 10 g/L chloralose (6 mL/kg, im)[4]. The abdomen of rats was open by the incision on the midline under the xiphoid. Small intestinal loops were pulled out and the mesentery was mounted on a hollowed transparent pedestal for observation. The specimen was suffused with a balanced 37 °C Kreb’s solution to maintain relatively normal condition in temperature and environment.
An Olympus microscope with a halogen lamp and Leitz long distance lens (20 × ) was used to observe the third order arterioles and venules. Being transmitted through a low-light level camera of model 1319, the signal was displayed on a Hitac hi color monitor. A JVC recorder was used for off line measurement. Each specimen was recorded no more than 30 min so as not to affect the mesentery microcirculation[3]. The MIMPCAS was used to measure the diameter (D) of blood vessels, the velocity of red blood cells (Vrbc), the visible leukocyte velocity (VLV), the visible leukocyte flux (VLF) and the adhesive leukocyte count (ALC) following the procedure described previously[4].
The following formula was used to calculate the parameters of microcirculation[4,6,9]: 1. mean blood velocity, Vmean = Vrbc/1.6; 2. flow volume of blood, F = Vmean×π×D2 / 4; 3. shearrate, γw = 8 × (Vmean / D); 4. total leukocyte flux, TLF = (60 ×F) ×K× 10-6, K is the amount of leukocytes in the blood[7]; 5. invisible leukocyte flux, ILF = TLF-VLF.
The results were represented by mean ± standard deviation (-x±s) and the significance of difference was judged by Student’s t test.
Twenty arterioles with a diameter between 11 µm-45 µm were selected for observation. Only one leukocyte rolled along the wall an d there was no leukocyte sticking on it in the third order arterioles. In the 20 capillaries with an average diameter of 6.3 µm ± 1.7 µm, the visible leukocyte flux was 0.2 cells/min ± 0.4cells/min and there were no plugging leukocytes. In the 20 venules with a diameter of 10 µm-50 µm, the visible leukocyte flux was 13.3 cells/min ± 7.2 cells/min and there were about 0.3 ± 0.3 leukocytes sticking on the wall within a length of 94 µm of blood vessels (Table 1). Under the physiological condition, the flow and distribution of leukocytes in different blood vessels varies to a large extent. The visible leukocyte flux (VLF) differed significantly between the venules and arteries with comparable diameter and blood velocity and the value of VLF in venules was as high as 131 times that of arterioles. Due to the interaction of leukocyte and endothelium which mainly occurred in venules, further studies were curried out on the flow and distribution of leukocytes in mesentery venules.
| Arteriole | Capillary | Venule | |
| Number | 20 | 20 | 20 |
| D(µm) | 22.6 ± 9.1 | 6.3 ± 1.7 | 25.1 ± 10.6 |
| Vmean(mm/s) | 1.07 ± 0.3 | 0.45 ± 0.12 | 0.86 ± 0.27 |
| Flow(pL/s) | 440.1 ± 439.2 | 14.1 ± 6.7 | 430.7 ± 412.4 |
| γw(s-1) | 371.1 ± 212.8 | 570.4 ± 189.7 | 272.1 ± 189.2 |
| TLF(cells/min) | 91.1 ± 90.9 | 2.9 ± 1.4 | 89.2 ± 85.4 |
| VLF(cells/min) | 0.1 ± 0.2b | 0.2 ± 0.4 | 13.1 ± 7.2b |
| ILF(cells/min) | 91.0 ± 90.9 | 2.7 ± 1.3 | 76.1 ± 77.8 |
| ALC(cells/94 µm) | 0 | 0 | 0.3 ± 0.3 |
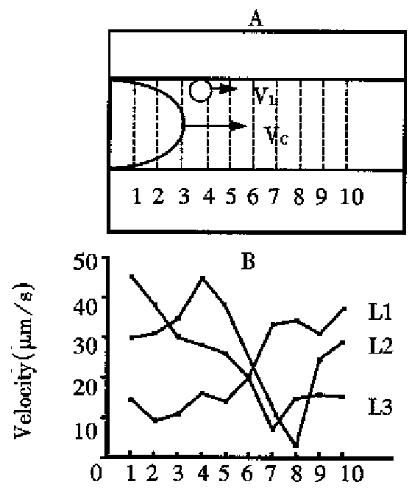
The space characteristic of the flow and distribution of visible leukocytes Ten sampling lines on vessel with equal distance were set perpendicular to the longitudinal vessel and the velocity of leukocyte passing through each line was measured. The variation of the velocity of leukocyte reflected the characteristic of its temporal distribution. Each leukocyte passing through the sampling lines with a large variation on velocity suggested that leukocyte rolling along the wall of blood vessels took a “jerky” movement (Figure 1). However, the average velocity for a leukocyte passing through a vessel with a definite length was similar, about 20 µm/s.
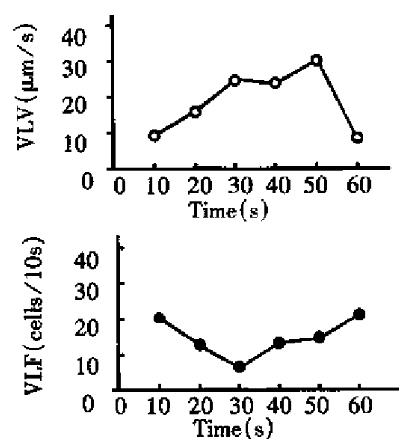
The time characteristic of the flow and distribution of visible leukocytes For the measurement, one line was set on a third order venule (D: 37 µm) of mesentery of rat . The velocity and flux of all the visible leukocytes passing the measuring line were determined in 10 s as one unit, and measurements were continuously performed 6 times in 1 min. It was found that visible leukocyte velocity and visible leukocyte flux changed temporarily (Figure 2).
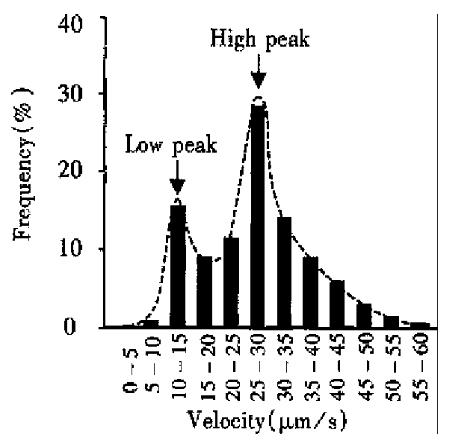
Frequency distribution of VLV The velocities of 400 visible leukocytes in 30 third branch venules were measured, of which the frequency distrib ution is shown in Figure 3. The frequency distribution of VLV presented with the characteristic of double peaks. Low peak value was about 10 µm/s-15 µm/swhile the high peak was around 25 µm/s-30 µm/s.Leukocytes with velocity below 5 µm/s or above 50 µm/s were rarely found.
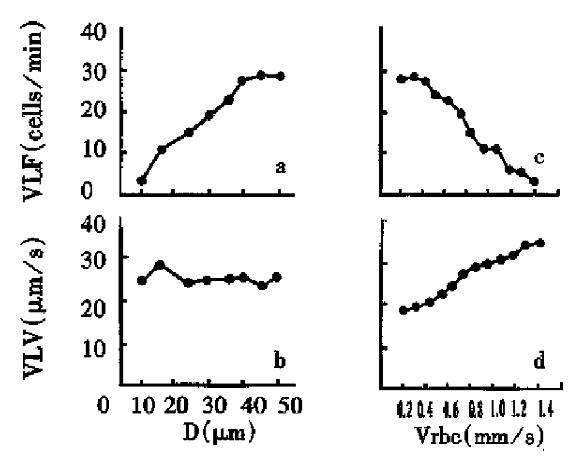
Visible leukocyte flux and visible leukocyte velocity were measured in 20 capillaries of mesentery of rats. Eight venules with similar blood velocity (Vrbc: 1.2 mm/s ± 0.1 mm/s) and vessel diameter (D: 10 µm-50 µm) were selected for the observation of influence of vessel diameter on the flow and distribution of leukocytes. It was found that with the increase of blood vessel diameter, visible leukocyte flux increased while visible leukocyte velocity remained relatively stable. Twelve venules with similar diameter (31.5 µm ± 1.1 µm) were selected for the observation of the influence of blood velocity on the flow and distribution.The blood velocity in these vessels ranged from 0.27 mm/s to 1.38 mm/s. Following the increase of Vrbc, visible leukocyte velocity increased while visible leukocyte flux decreased as shown in Figure 4.
Recently, wide interest has been shown in the research of leukocyte-endothelium interaction. Studies on the rheological behavior of leukocytes in the microcirculation have promoted the understanding on the mechanism of cellular adhesion. These studies mainly involved two aspects, i.e., one is the interaction between leukocytes and red blood cells and the other is that between leukocytes and endothelial cells[6,7].
It was found in this study that leukocytes scarcely rolled on the wall or firmly sticked in arterioles. Under normal condition, sticking leukocytes in venules were rarely observed while some leukocytes rolled along the vessel wall in the marginal stream, suggesting that the flow and distribution of leukocyte in venules and arterioles was significantly different. There exist some interactions between leukocytes and endothelium in venules, for which the major phenomenon is the leukocytes rolling on the endothelium of venule wall. The development of leukocyte rolling and sticking on endothelium depends on two forces: leukocyte-endothelium adhesive forces and hemodynamic dispersal forces, i.e., shear stress[1,8]. Mayrovitz had suggested that the adhesion of leukocytes on the vessel wall might be mainly related to shear stress, for the adhesion of leukocytes existed on the wall of post-capillary venules[7]. However, the results herein showed that VLV varied to a large extent even in the arterioles and venules with a similar shear stress, suggesting that under physiological condition the difference of leukocyte flow and distribution in different vessels mainly came from the characteristic of endothelial cells and the micro-environment around leukocytes[9].
The rolling of leukocytes along the walls presented with an uneven “jerky” movement. The balance between adhesive forces and dispersal forces was broken by the non-homogeneity of endothelium and that of hemodynamic forces, which brought about the rolling of leukocytes with the characteristic of non-stable speed[3,8,10]. The factors that influence homogeneity of endothelium include non-even surface of endothelium, local characteristic of endothelium, surface distribution of charge, the concentration of reactive substances, etc., while that for hemodynamic force, were temporal variation of blood velocity and local concentration of red blood cells. The results from the analysis on the frequency of VLV showed that the rolling leukocytes with a velocity lower than 10 μm/s had a potential to stick on the endothelium of blood vessels, while the rolling leukocytes with a speed higher than 30 μm/s tended to merge into the central stream of blood. The two peaks of the distribution of VLV suggested that there were at least two kinds of adhesive molecules with different property, by whi ch two different velocities of leukocyte rolling along the walls were mediated. However, the adhesive molecules involved in the leukocyte-endothelium interaction are waiting to be identified and it is also necessary to pay more attention to the study on the mechanism of cellular adhesion.
In this study, the impact of diameter of blood vessels and blood flow of venules on the flow and distribution of leukocytes was analyzed. The diameter of blood vessels were found to have a significant effect on the flow and distribution of leukocytes. In the blood vessels with a larger diameter, visible leukocyte flux (VLF) increased significantly, but the visible leukocyte velocity (VLV) kept stable. Atherton had suggested that the temporal contact between leukocyte and endothelium should be taken as an inelastic collision[3]. The increase of adhesion force would bring about more chances of random collision and higher degree of inelastic collision between leukocytes and endothelium. Visible leukocyte flux mainly reflects the random collision between leukocyte and endothelium, while visible leukocyte velocity indicates the degree of inelastic collision. In the larger vessels, the leukocyte flux and the area of endothelium are also larger, so the random contact chances increase to bring about high flux of visible leukocyte. The change of diameter would not impact the adhesion force, so visible leukocyte velocity was the same.
Given a certain extent of blood viscosity, shear stress is determined by blood velocity under the condition of definite vessel diameter[2,6,8,10]. The shear stress is high in the blood vessels with fast flow of blood, which will reduce the chance of collision between leukocyte and endothelium. Therefore in the vessels with fast blood flow, visible leukocyte flux is low while visible leukocyte velocity is high.
In summary, the flow and distribution of leukocytes in the mesentery microcirculation of rats was studied in vivo, and the influencial factors on which were explored under normal conditions. The result of this study is helpful for the understanding of the mechanism for leukocyte endothelium interaction in physiological state and provides theoretic basis for the study and treatment of increased LEI in pathological processes.
Project supported by National Natural Science Foundation of Chin a, No. 39270852.
Edited by Jing-Yun Ma
| 2. | Zhao K, Wu KY, Zhu ZJ, Huang XL. The role of leukocyte in the disorder of microcirculation during shock. Natl Med J Chin. 1986;66:722-725(Chin). |
| 3. | Atherton A, Born GV. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972;222:447-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 256] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Jiang Y, Zhao KS, Li SX. Computer assisted analysis of leukocyte rheological behavior in microvasculature. Chin Med J (Engl). 1993;106:883-888. [PubMed] |
| 5. | Zeintl H, Sack FU, Intaglietta M, Messmer K. Computer assisted leukocyte adhesion measurement in intravital microscopy. Int J Microcirc Clin Exp. 1989;8:293-302. [PubMed] |
| 6. | House SD, Lipowsky HH. Leukocyte-endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc Res. 1987;34:363-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 239] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Mayrovitz HN, Kang SJ, Herscovici B, Sampsell RN. Leukocyte adherence initiation in skeletal muscle capillaries and venules. Microvasc Res. 1987;33:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Zhao KS. Cytorheology-A new project for shock research. Med J Chin PLA. 1986;11:137-139 (Chin). |
| 9. | Schmid-Schönbein GW, Usami S, Skalak R, Chien S. The interaction of leukocytes and erythrocytes in capillary and postcapillary vessels. Microvasc Res. 1980;19:45-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 216] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Jones DA, Smith CW, and McIntire LV. Effect of fluid shear stress on leukocyte adhesion to endothelium cells. In: Granger DN and Schmid-Schonbein GW, eds. Physiology and pathophysiology of leukocyte adhesion. 1st ed. New York: Oxford University Press. 1995;148-168. |