Published online Jun 15, 1999. doi: 10.3748/wjg.v5.i3.217
Revised: February 21, 1999
Accepted: March 10, 1999
Published online: June 15, 1999
AIM: To explore the expression of perforin and granzyme B genes mRNA to judge the effect of immunosuppression in acute rejection of liver transplantation.
METHODS: The expression of perforin and granzyme B genes mRNA was examined by reverse transcription-polymerase chain reaction (RT-PCR) in hamster to rat liver grafts under the immunosuppression of cyclosporine or/and splenectomy. Histological findings were studied comparatively.
RESULTS: Cyclosporine could obviously decrease the cellular infiltration, and completely repress the expression of mRNA for perforin and granzyme B, but could not change severe hepatocyte necrosis and hemorrhage. Splenectomy could significantly lighten hepatocyte necrosis, and completely eliminate hemorrhage, but not affect the cellular infiltration and the expression of perforin and granzyme B genes mRNA. Cyclosporine or splenectomy alone could not prolong the survival time, however, their combination could completely repress the rejection of liver grafts. The survival time of animals were significantly prolonged (37.1 days). The architecture of hepatic lobules was preserved. There wes slight cellular infiltration in the portal tracts and no expression of perforin and granzyme B genes mRNA could been seen in three weeks after transplantation.
CONCLUSION: Perforin and granzyme B genes are valuable in judging the effect of immunosuppression in liver transplantation.
- Citation: Zhang SG, Wu MC, Tan JW, Chen H, Yang JM, Qian QJ. Expression of perforin and granzyme B mRNA in judgement of immunosuppressive effect in rat liver transplantation. World J Gastroenterol 1999; 5(3): 217-220
- URL: https://www.wjgnet.com/1007-9327/full/v5/i3/217.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i3.217
Rejection is one of major factors influencing the outcome of the patients after liver transplantation, and acute rejection is more harmful to the grafts and recipients. The cellular immunity has been proved to be a chief mechanism in rejecting liver transplantation, and cytotoxic T lymphocyte (CTL) is the major effector cell, the perforin lytic pathway to granzyme B, plays a critical role in the T-cell immun e response [1]. It is a hot issue of the moment to search for special early markers to judge the effect of immunosuppression in acute rejection of liver transplantation. In this study, the expression of perforin and granzyme B mRNA was examined by reverse transcriptase-polymerase chain reaction (RT-PCR) under the immunosuppression of Cyclosporine (CsA) and splenectomy based on the establishment of a stable and reliable model of hamster to rat concordant xenogeneic orthotopic liver transplantation.
Female golden hamsters weighing 150 g-180 g were the donors of liver xenografts, and male Wistar rats weighing 230 g-260 g were the recipients. The animals were purchased from Xi Bi Experimental Animal Centre and Shanghai Experimental Animal Centre of Chinese Academy of Sciences.
Orthotopic liver transplantation was performed according to simplified three-cuff technoque[2] with some modifications. Donor cholecystectomy was perfo rmed at the time of cuff preparation, without reconstruction of hepatic artery, and splenectomy at the time of transplantation with simple ligation of the splenic hilum and excision. No microscope was used for all the operations.
Liver xenografts were studied in four groups: A,untreated controls (n = 8); B, treated with cyclosporine 30 mg/kg/daily (n = 6); C,treated with splenectomy (n = 6); D, treated with splenectomy and cyclosporine 30 mg·kg·day (n = 7).
CsA was administered to recipients intramuscularly beginning on the first day of operation, at an interval of 12 h. Splenectomy was done at the time of transplantation.
Postoperative specimens at rejection and specimens taken at sacrifice were fixed in 10% formalin and stained with hematoxylin and eosin.
Total RNA was extracted according to the Qiagen kit directory. In brief, after homogenization and lysis with lytic buffer RLT in QIA shredder, same volume of 70% ethanol were added. Samples were then moved into RNeasy spin column and centrifuged for 15 s at 8000 × g, buffer RW1 and buffer PRE were added and centrif uged for 15 s at 8000 × g step by step. Finally, RNA was eluted with diethylpyrocarbonate (DEPC)treated water and centrifuged for 1min at 8000 × g. The approximate quantity of RNA was determined with an OD 260 nm and the purity was confirmed with an OD ratio of 260 : 280 to be greater than 1.8 in all spec imens. RNA was extracted from rat spenocytes stimulated in culture for 18 h with PHA (10 mg/L), Con A (10 mg/L) and IL-2 (20 units/mL) for the positive control. The RNA of normal rat liver served as negative control.
The cDNA synthesis was performed with GIBCOL BRL kit. RNA mixtures were prepared as follows: 2 µg of total RNA and 0.5 µg of oligo (dT) 12-18, were added with DEPC-treated water and diluted to 12 µL, water bathed at 70 °C for 10 min and incubated on ice for at least 1min, then added with 2 µL of 10 × PCR buffer, 2 µL of 25mM MgCl2, 1 µL of 10 mM dNTP, and 2 µL of 0.1 M DTT and incubated at 42 °C for 5 min. 200 U Super Script II RT was added and incubated at 42 °C for 50 min and the reaction was terminated at 70 °C for 15 min and chilled on ice, and finally 1 µL of Rnase H was added to each tube and incubated for 20 min at 37 °C. The cDNA was stored at -20 °C.
The DNA was amplified using RT-PCR on a Perkin-Elmer 2400 thermocycler, and RT-PCR primer was designed according to the exon of rat gene sequences. The primer set for perforin was (1) 5’GCCATCCTGCGTCTGGACCTG3’, (2) 5’CATTTGCGGTGCACGATGGAG3’; primer set for the granzyme was (1) GACTTTGTGCTGACTGCTGCTCAC3’, (2) 5’TTGTCCATAGGAGACGATGCCCGC3’; and for the β-actin: (1) 5’TGCTACACTGCCACTCGGTCA3’, (2) 5’GCATGCTCTGTGGAGCTGTTA3’[3]. The reaction mixture conta ined 2 µL cDNA, 1 µL of 10 mmol/L dNTP, 3 µL of 25 mmol/L MgCl2, 5 µL of 10 × buffer, 1 µL Taq polymerase, 2 µL each of the forward and reverse primer, and 34 µL of dual-distilled water for each 50 µL amplification reaction. Reactions were performed for 30 cycles. The conditions were 94 °C for 3 min prior to cycling, denaturing at 95 °C for 15 s, annealing at 60 °C for 20 s and extension at 70 °Cfor 30 s. Following amplification, ten µL PCR products were run on a 1.5% agarose gel stained with ethidium bromide, gene specific bands were visualized by phot ography under UV fluorescence.
Graft survival is shown in Table 1. Groups A, B and C showed rejection in 6-9 days. Groups B and C had rejection with a time course similar to group A (P > 0.05). Survival in group D was significantly prolonged to 37.1 ± 9.9 days ( P > 0.01).
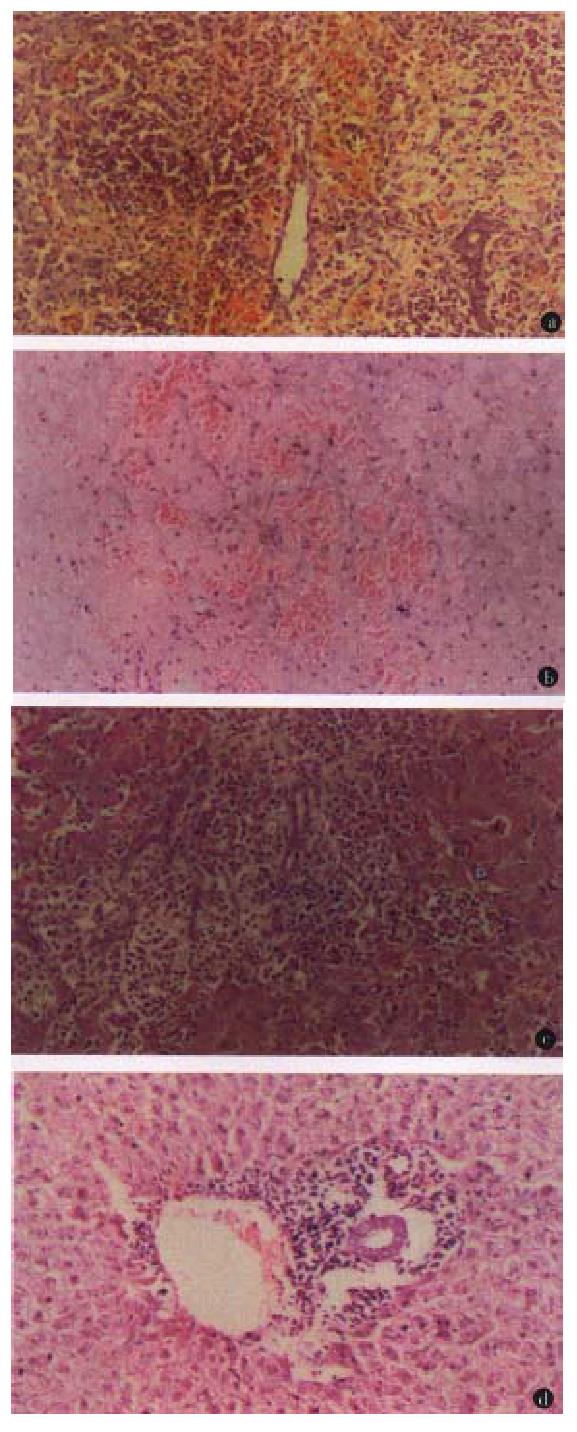
The liver xenografts in group A showed diffuse mononuclear cell infiltration, massive necrosis and interstitial hemorrhage (Figure 1A). In group B, CsA at dose of 30 mg·kg·day obviously decreased cellular infiltration, but severe hepatocyte necrosis and hemorrhage remained unchanged (Figure 1B). Splenectomy (group C) significantly alleviated hepatocyte necrosis and hemorrhage, but did not change diffuse mononuclear cell infiltration (Figure 1C). In group D, CsA and splenectomy abated cellular infiltration and hepatocyte necrosis and hemorrhage, the architecture of the hepatic lobule was preserved, but there was slight cellular infiltration in the portal tracts (Figure 1D).
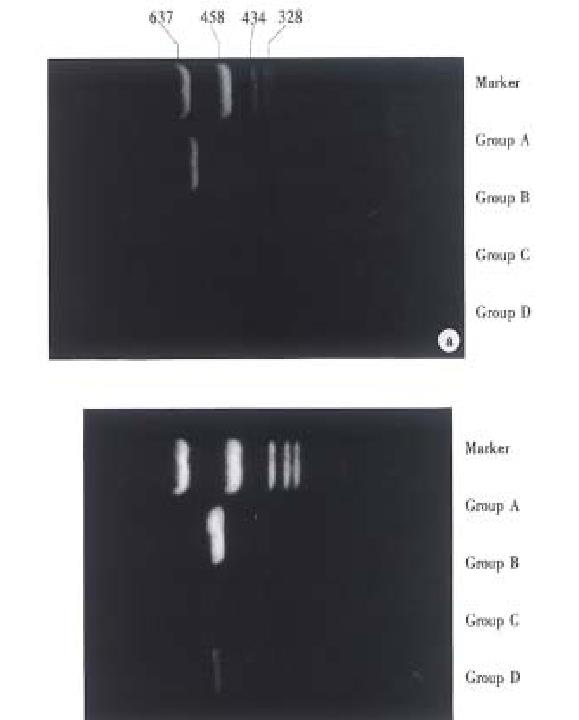
All recipients had the expression of perforin and granzyme B genes mRNA in group A on post-transplantation day (POD) 5; only one (1/6) in group B expressed mRNA of these genes on POD 5; and five recipients (5/6) in group C expressed mRNA of both genes on POD 5. There was no expression of both genes in group D on P OD 5 and 14, but only one recipient (1/7) expressed mRNA of perforin and granz yme B genes on POD 21 (Figure 2).
Gold hamster to rat orthotopic liver transplantation is concordant and heterotra nsplantation and presents with acute rejection. The recipient’s survival can not prolong until both cellular and humoral rejection are depressed due to its dua l-immune mechanism. Splenectomy can effectively inhibit antibody formation, obviously abated hepatocyte necrosis and hemorrhage, but is unable to improve the diffuse mononuclear cellular infiltration in the grafted liver. CsA can significantly decrease cellular infiltration, but can not improve hepatocyte necrosis and hemorrhage, neither of them can prolong the recipient’s survival when used alone, but they can inhibit both cellular and humoral rejection, normalize the architecture of the grafted liver, and significantly prolong the recipient’s survival to 37.1 days when used in combination. The result is better than that reported abroad[4].
CTL is believed to play an important role in the mechanism of rejection, and effect mechanism of perforin and granzyme B, in spite of the regulatory and effect mechanism underlying the rejection process, remains incompletely understood. Effect of immunosuppression on the expression of perforin and granzyme B mRNA has become a hot topic in recent years. Mueller et al[5] analyzed the expression of perforin and granzyme A genes in situ hybridization in cellular infiltrates of MHC mismatched mouse heart transplants both in immunosuppressed recipients treated with CsA and untreated recipients. In untreated grafts, there were many perforin and granzyme A-expressing cells and heart transplants were completely rejected on POD 10. In contrast, CsA treatment significantly decreased the positive cells and prolonged survival of the transplants to 30 days. CsA did not obviously decrease infiltration of CD8+ cells but significantly reduced the number of perforin and granzyme A-positive cells. It shows that CsA treatment mainly depressed the activation of CTL rather than decreased the number of infiltrating cells. Rapamycin can completely block the expression of granzyme B gene in infiltrating cells of grafts, obviously prolong the survival of grafts[6].
Our experimental results show that combined CsA and splenectomy could effectively depress rejection in hamster to rat orthotopic liver transplantation. The architecture of the hepatic lobule was undamaged, and the survival was significantly prolonged, and there were no expression of perforin and granzyme B genes mRNA.
In conclusion, expression of the CTL-associated gene perforin and granzyme B provides two valuable markers to judge the effect of immunosuppression in acute rejection of liver transplantation. But this should be further confirmed clinically.
Project supported by the National Natural Science Foundation of China, No. 39500152.
Edited by Jing-Yun Ma
| 1. | Griffiths GM, Mueller C. Expression of perforin and granzymes in vivo: potential diagnostic markers for activated cytotoxic cells. Immunol Today. 1991;12:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Zhang SG, Tan JW, Yang JM. Three cuff in hamster to rat ortho-topic liver transplantation. J Sec Milit Med Univ. 1998;19:89-90. |
| 3. | McDiarmid SV, Farmer DG, Kuniyoshi JS, Robert M, Khadavi A, Shaked A, Busuttil RW. Perforin and granzyme B. Cytolytic proteins up-regulated during rejection of rat small intestine allografts. Transplantation. 1995;59:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Valdivia LA, Monden M, Gotoh M, Hasuike Y, Kubota N, Ichikawa T, Okamura J, Mori T. Prolonged survival of hamster-to-rat liver xenografts using splenectomy and cyclosporine administration. Transplantation. 1987;44:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Mueller C, Shao Y, Altermatt HJ, Hess MW, Shelby J. The effect of cyclosporine treatment on the expression of genes encoding granzyme A and perforin in the infiltrate of mouse heart transplants. Transplantation. 1993;55:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |