Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.167
Revised: November 16, 1998
Accepted: January 10, 1999
Published online: April 15, 1999
-
Citation: Deng XZ, Diao ZY, He L, Qiao RL, Zhang LY.
HBeAg gene expression with baculovirus vector in silk worm cells. World J Gastroenterol 1999; 5(2): 167-171 - URL: https://www.wjgnet.com/1007-9327/full/v5/i2/167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.167
Miyanohara et al[1] first obtained products with the expression of HBeAg activity by constructing a yeast expression system; later researches discovered HBeAg expression in xeropus oocytes[2], COS cells[3], E. coli cells[4], Bacillus subtilis[5], which allow us to know more certainly about HBeAg gene. HBeAg has the same 149 amino acid sequence as the amino-terminal of HBcAg which is encoded by c-gene and consist s of 183 amid acids. The c-gene has a 89 bp pre-csequence in the upstream of it. The co-expressed product of the two genes (pre-c protein) is cleaved off the amino-terminal signal peptide sequence and the c-terminal alkaline region by hydrolases in the membrane of endoblasmic reticulum, forming the secretable HBeAg[6]. The most important thing is that the signal peptide encoded by pre-c region directs the formation and secretion of HBeAg, suggesting that only by eukaryotic expression systems can we produce HBeAg with high purity and activity. The domestic HBeAg/anti -HBe diagnostic kit is produced in E. coli cells, containing a high proportion of HBeAg which affects the quality of the kit. Recently, the technique with baculovirus vector to express foreign gene efficiently in worm cells and body has been applied and popularized[7]. We have replaced the polyhedron protein gene encoding sequence with human INF-α in Bombyx morinuclear poly-hedrosis virus( Bm NPV), suggesting that silk worm cells can recognize the signal peptide of human INF-α gene and cut correctly[8]. Ninety-nine percent protein becomes ma-ture only after the secreting stage, the Bm NPV-Bm N will be a good expression system. For this purpose, we amplified the pre-csignal peptide sequence and the same 149 amino acids sequence homologous with HBcAg at the N-end by PCR, and added appropriate restriction endonuclease sites on both 5’ and 3’ ends, cloned it into-Bm-NPV transfer vector pBmo30, the Bm-N cells were co-transfected by p-Bm-HBe and wild-type Bm-NPV DNA, and at length the recombinant virus with high expression HBeAg were efficiently obtainable after plaque purification.
Bm NPV transfer vector pBmo30 and silk worm cells were supplied by Virus Rese arch Institute of Wuhan University. The cells were cultured in Tc-100 (containing 100 mL/L-fetal calf serum) and then stored frozen. HBeAg gene was generated by PCR from the template DNA obtained from the HBV Library of the Virus Research Institute of Wuhan University[9].
The transfer vector pBmo30 and recombinant vector p Bm HBe DNA were extracted from E. coli cells by ordinary method. The silk worm cells were infected by BmNPV DNA, then cultured for 5-7 days at 27 °C, centrifuged at low speed when nuclear polyhedrons emerged. The supernatants containing virus particles were harvested to infect Bm N cells again, and the cells were cultured, observed as before, and centrifuged to maintain the cells and supernatants. The Bm N DNA was extracted from polyhedrons and viruses according to Summers program. The amplified fragments and enzyme-excised fragments were subjected to 7 g/L-10 g/L agarose gel electrophoresis respectively, and recovered by DE81 membrane method[10].
At the 3’ and 5’ ends of the HBV e gene, we took artificially synthesized 30 bp sequence as the primers. Bgl II locus was added to the (+) 5’ end of the primer, and xba I-TAA-Sma I locus to (-) 5’ end of the primer.
Primer 1(+): 5’AGATCTCATGGAACTTTTTACCTCTGCCT3’
Primer 2(-): 5’CCCGGGTTATCTAGAAACAACAGTAGTTTCCGGAA3’
PCR reaction was performed from the template PHB24 plasmid containing entire genic HBV and the above primers. PCR product was subjected to 7 g/L-agarosegel electrophoresis .
DNA sequence was analyzed to identify the amplified fragment through ddNTP/PCR/silver-stained sequence analysis system. PCR amplification was performed under the template DNA of purified 537 bp fragment, with the same primers, and under the presence of one type ddNTP according to silver-stained sequence a nalysis protocol. The samples were subjected to the 80 g/L PAG gel electrophoresis, then fixed, stained and colorized and the DNA sequence was read up.
The plasmid pBm DNA and PCR fragment were digested respectively by Bgl II and Sma I, ligated, then transfected into competent E. coli cells. The resistance colonies were selected from ApILB plates. Bgl II and Sma I digested the extracted recombinant DNA, the DNA samples were subjected to 100 g/L-agarose gel electrophoresis to identify the positive recombinant.
The extracted recombinant transfer vector DNA and wt Bm NPVDNA were mixed by 5:1 molar ratio, and then co-transfected the fresh growing well wall-adhering-Bm-N cells, through the mediation of liperfectin as previously described. 2 h later the medium was removed, and TC-100 (containing 100 mL/L- fetal calf serum) was added and the cells were cultured for 7-10 days at 27 °C. Cells containing recombinant viruses were chosen with plaquepurification on agars plates. Those plaques of 0- (occlusion) phenotype without polyhedrons, which were the positive recombinant viruses, were selected.
Bm N cells were infected by recombinant viruses, cultured for 4 days at 27 °C, centrifuged to get cells and supernatants, a 50 g/L SDS-PAG electrophor esis was performed as general method, stained with Cormassie blue, and the prote in expression was observed.
Cells were lysed with guanidine hydrochloride to rupture cell membrane and centr ifuged to get supernatants. Anti-HBe/HBcAg kit from Medicine Research Institute of Nanjin was used to perform ELISA, separately by using the HBeAg-positive serum of HBV patients and HBcAg generated from engineered bacteria as positive controls, and by using reptured -Bm-N cell medium containing normal receptor and cultured supernatant as negative controls. P, N values were calculated on the basis of OD value (A), P/N ≥ 2 was considered as positive.
Cell culture supernatants were collected, precipitated by 27% ammonium sulfate, and then dissolved by PBS (0.02 mol/L-PB, pH 7.0, 0.03 mol/L- NaCl). After separation from pre-balanced sephacryls-200 (1 cm × 100 cm) column, electrophoresis and ELISA detection were performed. The peak HBeAg-activity was captured. After gradient elution through DEAE-Sepharose FF ion exchange column, detection of HBeAg activity and SDS-PAG electrophoresis were performed. The general pressured liquid chromatographic system used was from Pharmacia Co.
The purified HBeAg expressed in silk worm cells was used to coat the enzyme labeled reaction plate (100 ng/well), incubated throughout the night at 4 °C, then serum to be detected was added to it, and after 30 min at 40 °C, the sametype of HBeAg was added to it. At last, TMB H2O2 was used to colorize it. Those P/N ≥ 2.1 were positive.
A series of PCRs were performed with the synthesized primers and plasmid PHB24 as template DNA; each PCR generated a fragment about 0.5 kb that was homolo gous with the HBeAg gene, within the 361 bp sequence from 5’end analyzed except one site (the 375, T→A), by using ddNTP/ PCR/silver staining. The 88 bp sequence from 273-361 of the amplified fragment was identified as HBeAg gene (Figure 1). The amplified HBeAg gene was 537 bp from the 5’signal peptide sequence. We designed Bgl-II site at 5’end and Xba-I, Sma-I si tes at 3’end for cloning.
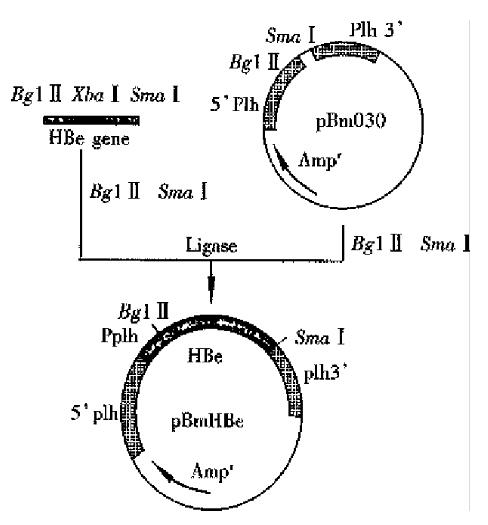
The Bm NPV transfer vector pBm 030 was 6.3 kb, containing polycloning site. pBm 030 DNA and the amplified fragment by PCR was digested by Bgl II/Sam I respectively, ligated by T4 DNA ligase, and allowed the e gene to be inserted into the ploycloning site under the control of plh promoter. The constructive processes was shown in Figure 2. The ligated DNA was transferred into E. coli cells, and positive colonies were selected. Bgl II/Sma I were used to digest the recombinant vector, and a fragment of 0.5 kb was obtained on agarose gel electrophoresis, indicating that HBeAg gene cloning was successful. Constructed recombinant viruses carried HBeAg gene.
Polyhedrosis observed in most cells was the signal of successful co-transfection. Other cells turned to have pathologic characteristics of infection, such asenlargement of cells and their nuclei, condentation of intracellular contents, and irregular granules. Polyhedrosis was not the typical characters of infection in recombinant viruses. So, after co-transfection gene recombination has completed between both plh gene on the 3’, 5’ ends of pBm Be DNA and the homol ogous gene of the wt Bm NPV plh gene. The plh gene was exchanged for the Pplh/HBe gene expression box, as controlled by plh promoter . The recombinant virus by plaque-purification was named r-Bm-HBe(Figure 3).
The silk worm cells were infected by recombinant virus rBmHBe, cultured for 72 h. And the cells and supernatants were harvested for SDS-PAG electrophoresis (Figure 4). The wt Bm NPV could produce polyhedron protein (Mr 32000), while the recombinant virus rBm HBe produced HBeAg about Mr 18000 instead of polyhedrosis because of the exchange of plh gene. The expression of HBeAg was also observed in the cultured medium, but with smaller molecular weight. So most of the expressed HBeAg was secreted out of cells induced by the signal peptide at N-end. ELISA was performed on culture cell lysate and cell culture supernatant to detect the activity of the expressed HBeAg (Table 1). A positive reaction can be found when the culture cell lysate was diluted 1:2000. The antigenic activity of the culture supernatant was much higher, reaching a dilution of 1:32000. Also no HBcAg was detected in the cell culture supernatant, and HBcAg in culture cell lysate was detectable only at a dilution lower than 1:160. The above results definitly proved that HBeAg antige nicity was expressed in silk worm cells.
| Sample | Dilution | ||||||
| 1:1000 | 1:2000 | 1:4000 | 1:8000 | 1:16000 | 1:32000 | 1:64000 | |
| Cell culture medium | + | + | + | + | + | + | - |
| Culture cell lysate | + | + | - | - | - | - | - |
The HBeAg expressed in silk worm cells was used to coat the enzymelabelled plate, then anti-HBe antibodies in samples were detected. The results were showed in Table 2.
Sephacryls-200 chromatography was performed firstly, and showed five protein peaks. ELISA detection indicated that most HBeAg existed in the fourth peak fraction. After concentration, DEAE-Sepharose FF chromatography was performed to get purified HBeAg. The recovery rate was about 52% (Table 3).
| Method | Total activity (by ELISA) | Specific activity (per mg protein) | Purification factor | Total recovery (%) | |
| A | B | ||||
| Cell culture medium | 1:1.60 × 106 | 1:2.9 × 105 | 1.0 | / | / |
| (NH4)2SO4 | 1:1.32 × 106 | 1:8.5 × 105 | 2.9 | 2.9 | 80 |
| Sephacryl S-200 | 1:1.12 × 106 | 1:5.3 × 104 | 6.3 | 18.3 | 70 |
| DEAE Sepharose FF | 1:8.40 × 105 | 1:5.0 × 105 | 9.4 | 172.0 | 52 |
The purified HBeAg expressed in silk worm cells and E.coli-cells was adjusted to a protein concentration of 2 g/L-each, then detected by HBeAg detection kit and detection anti-HBe antibody kit sallied in market (Table 4). Those values indicated in Table 4 were dilution magnitude; the P/N ≥ 2.1 wasused to determine the end point.
| Antigen | Cell | HBeAg kit | Anti-HBc kit |
| HBeAg 1 | karyotic | 10-7 | 10-1 |
| HBeAg 2 | E. coli | 10-5 | 10-3 |
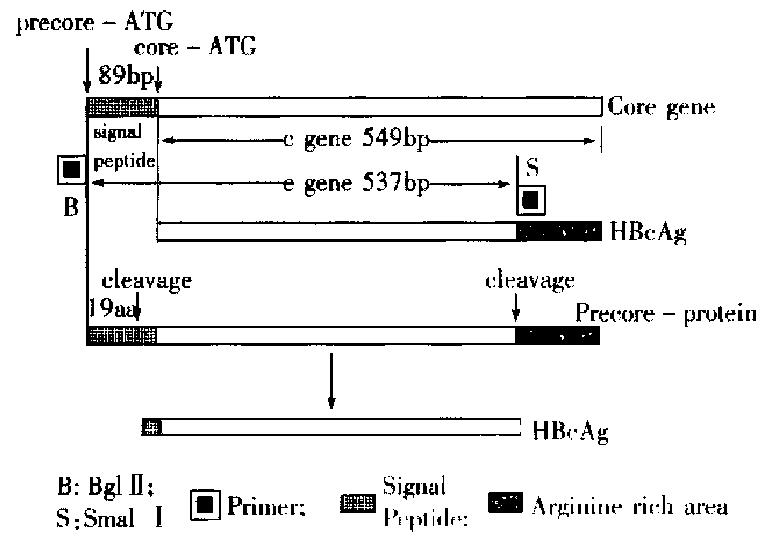
We constructed a transfer vector pBm HBe with HBeAg gene fragment from PCR. This vector was used together with wt Bm NPV to co-transfect the silk worm cells, and the recombinant viruses carrying HBeAg gene was obtained. The amplified HBeAg gene was 537 bp long, including an 89 bp sequence of signal peptide from 5’-end to 3’-end of e gene. The relation between c gene and it was shown in Figure 5. While HBeAg was expressed in silk worm cells, the signal peptide was clipped off in granular endoplasmic reticulum, and 19 amino acids were lost with the HBeAg secreting into cell cultured medium. And some antigens in non-secretary form also existed in cells. On the basis of gene sequence analysis, an ATG was found to locate at both 5’-and 3’-ends of the signal peptide, and the second ATG obeyed the Kozak rule completely. When 40s subgroup of ribosome was scanned to the first AUG codon, some of the 40s submits and 60 s submits would fit into ribosomes, begin to transcribe and produce protein carrying signalpeptide, which was processed and secreted out of the cells as soluble HBeAg. The HBeAg reaction rate was 100-fold higher, and the anti-HBc cross reaction rate was 100-fold lower, compared with the reaction using expression by prokaryotic cell in the same concentration of protein, because prokaryotic cell system did not differentiate and cut message peptide sequence. The products expressed were cellular c antigen (Table 4).
The other 40 s subgroup continued to scan until the second AUG, combined with the 60 s subgroup and began to transcribe, and produce protein without signal peptide, which remained inside of the cells. Such a result was consistent with the the orctic basis of the initial regulation of mRNA transcription[2]. The expressed product inside cells should be HBcAg according to the c gene sequence ana lysis, but ELISA results proved it was HBeAg (1:2000) mostly, with little HBcAg ( < 1:160). So the phenomenon suggested that the arginine abundant region at the carboxyl end should be very important for HBcAg expression; it took part in the self-fitting into core particle of HBcAg protein. The amplified e gene fragment did not contain the arginine abundant region, so its expressed product contained little HBcAg.
Edited by Han-Ming Lu
| 1. | Miyanohara A, Imamura T, Araki M, Sugawara K, Ohtomo N, Matsubara K. Expression of hepatitis B virus core antigen gene in Saccharomyces cerevisiae: synthesis of two polypeptides translated from different initiation codons. J Virol. 1986;59:176-180. [PubMed] |
| 2. | Standring DN, Ou JH, Masiarz FR, Rutter WJ. A signal peptide encoded within the precore region of hepatitis B virus directs the secretion of a heterogeneous population of e antigens in Xenopus oocytes. Proc Natl Acad Sci USA. 1988;85:8405-8409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Ou JH, Yeh CT, Yen TS. Transport of hepatitis B virus precore protein into the nucleus after cleavage of its signal peptide. J Virol. 1989;63:5238-5243. [PubMed] |
| 4. | Deng XZ, Zhang LY, He L, Zhang Z. High expression of Hepatitis B e gene in E. Coli. J China Pharmaceutical Univ. 1992;23:290-293. |
| 5. | Qi YP, Ma XW, Hung YX, Tan YP. Cloning and expression of hepatitis B virus e gene in bacillus cells. J Wuhan Univ. 1993;39:99-106. |
| 6. | Gerlich WH, Heermann KH. Function of hepatitis B virus protein and virus assembly. 1991;121-134. |
| 7. | Lu M, Iatrou K. Characterization of a domain of the genome of BmNPV containing a functional gene for a small capsid protein and harboring deletions eliminating three open reading frames that are present in AcNPV. Gene. 1997;185:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Deng XZ, Zhang LY, Yu MG, Li GF, Diao ZY, Zhong J. The replication of recombinant bombyx mori muclear polyhedrosis virus (r-Bm-NPV) and expression of Hu IFN-α gene. J Wuhan Univ. 1995;41:324-328. |
| 9. | Qi YP, Hung YX, Wei ZY. Construction of gene band for Hepati-tis B virus ayw subtype. J Wuhan Univ. 1989;112-116. |
| 10. | Simbraok J, Fritsch EF, Maniatis T. Molecular cloning, A labora-tory manual, 2nd ed. Cold Spring HarboAr Laboratory Press. 1989;776-786. |