Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.143
Revised: February 3, 1999
Accepted: February 14, 1999
Published online: April 15, 1999
AIM To investigate the relationship between alanyl-glutamine (ALA-GLN) and glutathione (GSH) biosynthesis in hepatic protection.
METHODS Twenty male Wistar rats were randomly divided into two groups: one receiving standard parenteral nutrition (STD) and the other supplemented with or without ALA-GLN for 7 days. The blood and liver tissue samples were examined after 5-fluorouracil (5-FU) was injected peritoneally.
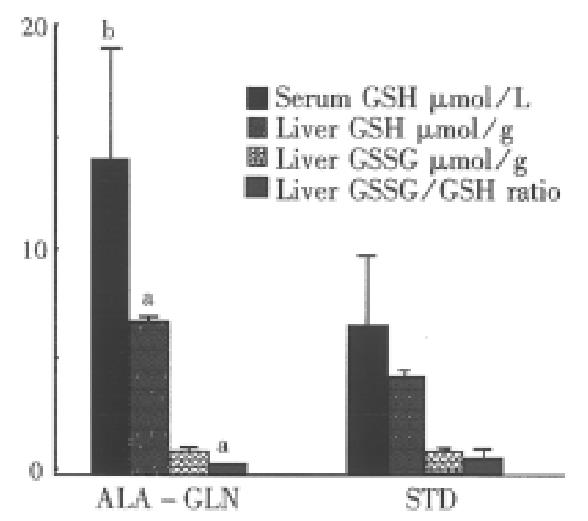
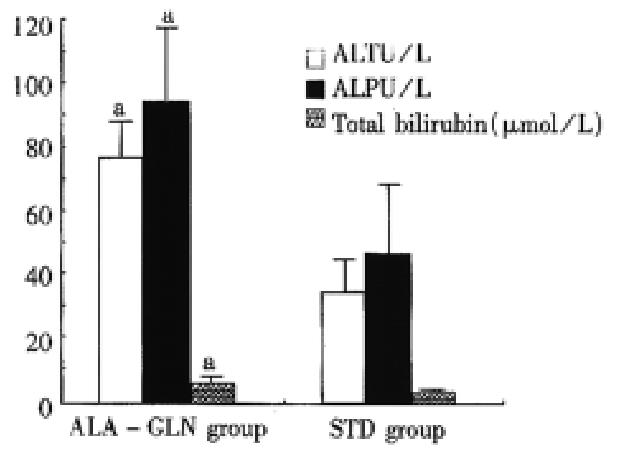
RESULTS The concentration measurements were significantly highe r in ALA-GLN group than in STD group in serum GLN (687 μmol/ L ± 50 μmol/L vs 505 μmol/L ± 39 μmol/L,P < 0.05), serum GSH (14 μmol/L ± 5 μmol/L vs 7 μmol/L ± 3 μmol/L, P < 0.01) and in liver GSH content (6.9 μmol/g ± 2.5 μmol/g vs 4.4 μmol/ g ± 1.6 μmol/g liver tissue, P < 0.05). Rats in ALA-GLN group had lesser elevations in hepatic enzymes after 5-FU administration.
CONCLUSION The supplemented nutrition ALA-GLN can protect the liver function through increasing the glutathione biosynthesis and pre-serving the glutathione stores in hepatic tissue.
- Citation: Yu JC, Jiang ZM, Li DM. Glutamine: a precursor of glutathione and its effect on liver. World J Gastroenterol 1999; 5(2): 143-146
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/143.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.143
Glutamine (GLN) was considered as a nonessential amino acid previously which is most abundant in the circulation system and in the amino acid pool of the body. The traditional standard amino acid solution does not contain glutamine since glutamine is unstable during sterilization at high temperature. In recent studies, GLN has also been demonstrated as a conditional essential amino acid, which plays a central role in the response to injury; and GLN also supports acid-base homeostasis[1], maintains the function and morphology of the gastrointesti nal epithelium[2,3], preserves the stores of antioxidants in tissues[4,5], enhances the immune response[6] and augments host defenses. The standard parenteral nutrition supplemented with GLN could significantly increase GSH stores and the survival rate of injured animal models. Therefore, GLN plays a major role in various anti-injury processes.
In this study, we used alanyl-glutamine dipeptide (ALA-GLN) solutions which are stable with the same biological effects as GLN and selected a model of 5-FU induced free radical-mediated hepatic injury, so as to establish a causal relationship between preservation of hepatic glutathione stores by glutamine administration and diminished hepatic injury caused by the toxicity of chemotherapy.
Male Wistar rats (211 g ± 16 g) obtained from Animal Laboratories of Chinese Academy of Medical Sciences were allowed to acclimatize for 5-7 days at constant temperature with a 12 h light/dark cycle. Only those animals demo nstrating normal food intake and weight gain were entered into the study. The rats were randomized to receive either the standard as control (STD, n = 10) or alanyl-glutamine (ALA-GLN, n = 10) parenteral nutrition. At the fifth day after catheterization 5-FU, a chemotherapeutic drug, was administered introperitoneally (100 mg/kg) for GSH depletion as a model of stress injury and intestin al inflammation. Animals were killed on the seventh day after catheterization for harvest of tissue and blood. Mortality rate was as-sessed.
The animals were anesthetized with intraperitoneal sodium pentobarbital (45 mg/kg). The hair was clipped from the cervical and interscapular regions, and the skin prepared with alcohol and povidone-iodine . Under sterile conditions, a Silastic catheter with an internal diameter of 0.76 mm and external diameter of 1.52 mm(Dow Corning Corp, USA) was inserted into the right jugular vein, advanced into the superior vena cava, and secured in place. The catheter was tunneled subcutaneously to the interscapular region and anchored to the deep f asiausing a stainless steel button and prolene (Ethicon, USA). After threading the catheter through a flexible wire sheath, the combined unit was attached to a swivel apparatus (Instech Laboratories, Inc. USA ) that allowed free movement of the animal during sustained intravenous feeding. After recovery from anesthesia, the animals were housed in individual metabolic cages.
Parenteral feeding solutions were prepared in a laminar flow hood and were sterilized using membrane filtration (0.22 μm filter). Stock solutions were prepared on the day of catheterization and stored at 4 °C. Both the stand ard glutamine-free parenteral solution (STD) and the 3% alanyl-glutamine dipep tide supplemented solution (ALA-GLN) provided the necessary calories, nitrogen, vitamins, and minerals required for the growing rats[7]. The diets were isocaloric (260 Kcal/kg/d, in which 20% Intralipid provides 20% calories) and isonitrogenous (1.66 g/kg/d), and differed only in the composition of alaninyl-glutamine dipeptide (alaninyl-glutamine 6 g + standard amino acid solution 100 mL + non-protein c aloric solution 100 mL, i.e. 3% ALA-GLN, equals to 2% GLN). After catheter ization and recovery from anesthesia, animals were randomized to receive either the STD (n = 10) or ALA-GLN (n = 10) parenteral nutrition at an initial rate of 24 mL/day. On the second day after the operation, the infusion rate was increased to 48 mL/day and maintained at this level throughout the experiment. At 08:00 am of the fifth day after catheterization, 5-FU was administered introperitoneally (100 mg/kg). Animals were killed on the seventh day after catheterization for harvest of tissue and blood. Mortality rate was assessed only in those animals killed at 24 h.
At the time of harvest, animals were weighed and anesthetized with introperitoneal sodium pentobarbital (45 mg/kg) and the skin prepared with povidone-iodine. Under sterile conditions, a midline incision was made from the pubis to the suprasternal notch, and whole blood was drawn from the right ventricle into a heparinized syringe. The whole blood was centrifuged immediately and plasma obtained for determination of glutathione, glutamine, alanine aminotransferase (ALT), alkaphospholinase (ALP), total and direct bilirubin concentration. The plasma (200 μL) added with 86.2 g/L-SSA (5-sulfosalicylic acid solution 200 mL) was centrifuged for 1 min immediately. The supernatant was removed and stored at -80 °C for determination of the reduced glutathione. A 400 mg liver without capsule was resected from the middle lobe and immediately placed in a 4.31% SSA solution with a weight to volume ratio of 1:10. These samples were then homogenized in a glass manual tissue grinder . The acid suspension was centrifuged and the supernatant sample was removed. For determination of disulfied glutathione (GSSG), an aliquot of previous supernatant was mixed with TRIS buffer and 2-vinylpyridine (Sigma Company) to protect the SH group directly during harvesting. Those samples were stored at -80 °C until analysis of the GSH and the oxidized form of glutathione (GSSG).
Biochemical assay of plasma and hepatocyte cellular glutathione was based on the “Glutathione, Glutathione Reductase-DTNB Recycling Method” originally descri bed by Tietze[8]: 2GSH + DTNB → GSSG + TNB + 2H+; GSSG + NADPH + H+→ 2GSH + NADP+.
The principle of the assay is illustrated as follows. GSH is oxidized by 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) (Sigma Company), yielding 2-nitro-5-thiobenzoic acid (TNB) monitored at 412 nm spectrophotometrically (UVIKON 930, KONTRON INSTRUMENTS); and GSSG is reduced by nicotinamide-adenine dinucleotide ph osphate (NADPH, reduced form, Sigma) in the presence of glutathione disulfide reductase. In the process, GSH is oxidized to GSSG, and the reaction is maintained by adding the enzyme glutathione reductase (Sigma Company) and the electron donor NADPH, so that the concentration of GSH is rate limiting. The GSSG was measured with the samples treated with 2-vinylpyridine masking GSH specifically. A standard curve with known concentration of glutathione was generated for each sample analysis. Plasma glutamine was determined with amino acid autoanalyzer (Model 119 CL, Beckman, USA). ALT, ALP and total and direct bilirubin concentrations were assayed using an automated laboratory analyzer (RA 2000).
All calculations were performed on an Apple Macintosh SE computer using STATVIEW standard statistical software. Data were expressed as mean ± standard error. Chi square analysis was used for mortality data. Other data were analyzed with ANOVA.
Although the body weight loss occurred during parenteral nutrition (PN) and 5-FU treatment, there were no significant differences in body weight between STD and ALA-GLN groups before starting PN(215.2 g ± 22.5 g vs 207.2 g ± 14.6 g, P > 0.05) or after finishing PN (188.1 g ± 19.9 g vs 190.6 g ± 19.8 g, P > 0.05).
48 h after 5-FU administration, animals in the experimental group had significantly higher plasma concentrations of GLN (687.3 μmol/L ± 49.8 μmol/L vs 504.9 μmol/L ± 39.6 μmol/L, P < 0.05) and GSH (14.37 μmol/L ± 5.16 μmol/L vs 7.08 μmol/L ± 3.16 μmol/L, P < 0.01) than in the control group (Figure 1).
48 h after durg administration, hepatic glutathione concentrations were significantly lowered in the STD group. But the GSSG level was similar between the two groups. The GSH concentration was in the normal range in the ALA-GLN group, but significantly higher than that in the STD group (6.9 μmol/g ± 2.5 μ[WTB1]mol/g vs 4.4 μmol/g ± 1.6 μmol/g liver tissue, P < 0.05). The redox ratio of GSSG/ GSH in ALA-GLN group was significantly lower than that in the STD group (0.12 ± 0.03 vs 0.47 ± 0.33, P < 0.05, Figure 1).
48 h after drug administration, STD animals had greater elevations in plasma ALT, ALP and total and direct bilirubin levels as compared with the ALA-GLN animals (P < 0.05) (Figure 2). One rat in ALA-GLN group and two rats in STD group died at 24 h after drug administration, and one rat in STD group died at 34 h after drug administration. Although mortality rate was higher in STD (30%) than in ALA-GLN animals (10%), there was no significant difference in mortality rate between the two groups (P > 0.05).
Glutathione (GSH) is a major antioxidant and a vital component of host defenses. It protects tissues from free radical injury via detoxification of active species and/or repair of injury, and plays an important role in the metabolism of drugs and endogenous substances[9]. The depletion of tissue GSH concentration and protein alkylation results in the drug toxicity of target tissues. The drug toxicity to normal tissues limits the chemotherapy doses.
GSH is a tripeptide consisting of glutamate, cysteine and glycine. Although experiments showed that GSH biosynthesis is rate-limited by cysteine during glutathi one depleted states[10], the availability of glutamine appears to be important for the re-generation of glutathione stores during our experiment of hepatic injury. The reason is that glutamate is poorly transported into cells. Under various experimental conditions, the glutamate portion of the molecule is derived from glutamine. Glutamine is efficiently transported across the cell membrane and deaminated in the mitochondria to produce glutamate and NH3. Glutamate is then transp orted back to the cytosol, where it is readily available for glutathione synthesis. GLN is a vital precursor of glutamate for GSH synthesis.
After 5-FU was administered in our study, STD animals exhibited a significantly lower glutathione stores as compared with that in the ALA-GLN group animals. The concentration of GSH which was in the normal range in GLN group was significantly higher than that in the STD group. 96 h after chemotheraputic drug administration, hepatic glutathione concentrations, GSH concentration and the GSH/GSSG-ratio in STD animals did not recover to the normal range. The possible reason may be the deficiency of GLN as a processor for GSH biosynthesis, limiting glutathione synthesis. This level of GSH depletion was associated with increased hepatic injury, as suggested by higher concentrations of hepatic enzymes. The normal concentration of GSH was associated with better hepatic function in the ALA-GLN animals.
These results suggest that 3% ALA-GLN has equivalent biological and metabolic effects with 2% GLN[11].The study confirms the important function of glutamine in the causal relationship between preservation of hepatic glutathione stores by glutamine administration and decrease of hepatic injury and mortality. This may be due to the mechanism of increased cellular reduced glutathione through the cell membrane [12]. GSH may also protect cells via detoxification by binding with the toxicant and repairing the injury of hepacytes.
We demonstrated that alanyl-glutamine could protect the liver function after chemotherapy through increasing the glutathione biosynthesis and preser ving the glutathione stores of hepatic tissue.
Edited by Jing-Yun Ma
| 1. | Welbourne TC, Joshi S. Interorgan glutamine metabolism during acidosis. JPEN J Parenter Enteral Nutr. 1990;14:77S-85S. [PubMed] |
| 2. | Hwang TL, O'Dwyer ST, Smith RJ, Wilmore DW. Preservation of small bowel mucosa using GLN-enriched parenteral nutrition. Surg Forum. 1986;37:56. |
| 3. | Rene RWJ, Bernard K, Maarten F. Glutamine and the preservation of gut integrity. Lancet. 1993;334:1364. |
| 4. | Wilmore DW, Smith RJ, O'Dwyer ST, Jacobs DO, Ziegler TR, Wang XD. The gut: a central organ after surgical stress. Surgery. 1988;104:917-923. [PubMed] |
| 5. | Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DW. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg. 1992;215:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 176] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Burke DJ, Alverdy JC, Aoys E, Moss GS. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg. 1989;124:1396-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 204] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Popp MB, Morrison SD, Brennan MF. Growth and body composition during long-term total parenteral nutrition in the rat. Am J Clin Nutr. 1982;36:1119-1128. [PubMed] |
| 8. | Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711-760. [PubMed] |
| 9. | Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163-L173. [PubMed] |
| 10. | Black M. Acetaminophen hepatotoxicity. Gastroenterology. 1980;78:382-392. [PubMed] |
| 11. | Jiang ZM, Wang LJ, Qi Y, Liu TH, Qiu MR, Yang NF, Wilmore DW. Comparison of parenteral nutrition supplemented with L-glutamine or glutamine dipeptides. JPEN J Parenter Enteral Nutr. 1993;17:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Le CT, Hollaar L, Van der Valk EJ, Franken NA, Van Ravels FJ, Wondergem J, Van der Laarse A. Protection of myocytes against free radical-induced damage by accelerated turnover of the glutathione redox cycle. Eur Heart J. 1995;16:553-562. [PubMed] |