Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.138
Revised: January 14, 1999
Accepted: January 26, 1999
Published online: April 15, 1999
AIM The food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) induces colon and mammary gland tumors in rats and has been implicated in the etiology of human colorectal cancer. This study was conducted to examine the potentially preventive effect of Chinese cabbage (Brassica chinensis), a brassica vegetable most commonly consumed in China, against this carcinogen induced DNA adduct formation in rats and its possible mechanisms. METHODS Sprague-Dawley rats were maintained for 10 days on basal diet or diet containing 20% (w/w) freeze-dried cabbage powder prior to administration of a single dose of PhIP (10 mg/kg) by oral gavage. Rats were sacrificed at 20 h after PhIP treatment and PhIP-DNA adducts in the colon, heart, lung and liver were analyzed using 32P-postlabeling technique. Levels of hepatic cytochrome P450 (CYP) 1A1 and 1A2, as indicated by 7-ethoxyresorufin O-deethylase and 7-methlxyresorufin O-demethylase activity, and cytosolic glutathione-S-transferases (GSTs) towards 1-chloro-2, 4-dinitrobenzene (CDNB) in the liver, lung and colon were measured.
RESULTS Rats pre-treated with Chinese cabbage and given a single dose of PhIP had reduced levels of PhIP-DNA adducts in the colon, heart, lung and liver, with inhibition rates of 82.3%, 60.6%, 48.4% and 48.9%, res pectively(P < 0.01). The enzyme assays revealed that Chinese cabbage induced both CYP1A1 and 1A2 activity, but the induction was preferential for CYP1A1 over 1A2 (81% vs 51%). GST activity towards CDNB in the liver and lung, but not colon, was also significantly increased by cabbage treatment.
CONCLUSION The results indicate that Chinese cabbage has a preventive effect on PhIP-initiated carcinogenesis in rats and the mechanism is likely to involve the induction of detoxification enzymes.
-
Citation: Tan W, Lin DX, Xiao Y, Ff K, Chen JS. Chemoprevention of 2-amino-1-methyl-6-phenyli-midazo [4, 5-
b ] pyridine-induced carcinogen-DNA adducts by Chinese cabbage in rats. World J Gastroenterol 1999; 5(2): 138-142 - URL: https://www.wjgnet.com/1007-9327/full/v5/i2/138.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.138
The incidence of colorectal cancer is generally lower in China as compared to Western countries, but has been increasing consistently and rapidly in urban population in the last two decades, presumably due to the change of lifestyle, particularly in dietary habits, e.g., increased consumption of animal foods[1]. It has been shown that a large number of heterocyclic amine carcinogens are formed in meat and fish during cooking. Among them, one of the most abundant heterocyclic amines, 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (Ph IP), has been proved to be carcinogenic for the colon and mammary gland of rats[2]. As several heterocyclic amines including PhIP have been detected in human urine after intake of normally cooked food[3], it would appear that humans are continuously exposed to these carcinogens from their diet. After metabolic activation via oxidation and esterification, PhIP binds to DNA to form PhIP-DNA adducts[4], which is widely believed to represent an important genotoxic step in the initiation of carcinogenesis. It has recently been found that PhIP-DNA adducts were present in surgical samples of human colon[5]. These findings strongly suggest the importance of PhIP in the etiology of human colorectal cancer. Epidemiological data also support this hypothesis, show ing that consumption of meat, especially well-done meat, is associated with an incre ased risk for human colorectal cancer[6]. In view of the fact that absolute avoidance of exposure of human to food-borne heterocyclic amines is impossible, seeking chemopreventive agents against these carcinogens is thus warranted.
The hypothesis that plant foods are protective against cancers at various sites has drawn interest in recent years and has been supported by many studies. A large number of potentially anticarcinogenic agents found in vegetables, may act as inhibitors in initiation, promotion and progression of the carcinogenesis. Brassica vegetables and their components are considered to be able to modulate expression of enzymes involving in metabolism of carcinogenic compounds, thereby preventing the carcinogenicity of the carcinogens[7]. Numerous epidemiological studies have consistently indicated that regular consumption of brassica vegetables reduces the incidence of human cancer including colorectal cancer[8]. Chinese cabbage (Brassica chinensis) is one of the most commonly consumed vegetables in China. In the present study, we examined the preventive effects of Chinese cabbage on PhIP-DNA adduct formation in rats and its possible mech anisms.
PhIP was purchased from Toronto Research Chemicals (Ontario, Canada). Resorufin, 7-methoxyresorufin and 7-ethoxyresorufin were obtained from Sigma Chemical Co mpany (St Louis, MO, United States). Reagents and materials for 32P-postlabeling analyses were from sources described previously [4] . Chinese cabbage was locally purchased and its fine powder was made by freeze-drying.
Weanling male Sprague-Dawley rats (146 g ± 15 g body wt) were obtained from Laboratory Animal Services, Chinese Academy of Medical Sciences (Beijing, China) and housed in a climate controlled room with a 12 h light/day cycle. Animals maintained on basal diet and tap water ad libitum for one week before experiment were randomly divided into three groups (5 rats/group). Group A and B were given basal diet, while group C was given basal diet containing 20% (w/w) Chinese cabbage powder. After 10 days on the diets, the animals in group B and C received a single dose of PhIP (10 mg/kg-body wt) by oral gavage administration. Control rats (group A) were given the same volume of 55% ethano l in saline (pH 4.5) used as vehicle for PhIP. Animals were killed under ether anesthesia at 20 h after PhIP treatment and the liver, lung, heart and colo n from each rat were removed. The tissues were immediately placed in cold saline, and then frozen quickly in liquid nitrogen and stored at -80 °C.
The liver and lung were thawed and washed at 4 °C with phosphate-buffered saline. The minced tissues were homogenized in an ice-cold Teflon-glass ho m ogenizer in 10 mmol/L-potassium phosphate bu ffer (pH 7.4) containing 1.4 mmol/L-β-mercaptoethanol and 0.25 mol/L-sucrose. The colon mucosal cells were removed from the tissues using a spatula and were homogenized as described for the liver and lung . Homogenates were centrifuged at 9000 g for 20 min, microsomes and cytosols were obtained by centrifuging the resulting supernatant at 105000 × g for 45 min. After collecting cytosol fractions, the microsomes were washed once by resuspension in 100 mmol/L-potassium phosphate buffer (pH 7.0) and followed by recentrifugation at 105000 × g for 45 min. The washed microsomes were resuspended in 3-weight volume of 100 mmol/L-potassium phos phate buffer (pH 7.0) containing 20% (v/v) gly cerol. Cytosols and microsomal suspensions were stored in aliquots at -80 °C until assayed.
DNA was isolated from each liver, lung, heart and colon, and PhIP-DNA adducts were analyzed using 32P-postlabeling technique as described[4] previously with minor modifications. Briefly, DNA samples were digested with micrococcal nuclease and spleen phosphodiesterase. The digests were then extracted with n-butanol and 32P-labeled with [γ-32P] ATP. After treatment with nuclease P1, the 32P-labeled adducts were separated by thin-layer chromatography (PEI-cellulose). Adducts were then detected using electronic autoradiography system (Packard Instrument Company, United States) with PhIP-modified calf thymus DNA as a standard.
Cytosolic glutathione S-transferase (GST) activity was determined with 1-chloro-2-dinitrobenzene (CDNB) as substrate as described by Habig et al[9]. The reaction mixture consisted of 1 mmol/L-GSH and 1 mmol/L-CDNB in 1mL of 100 mmol/L potassium phosphate buffer, pH 6.5. The reaction was started by additing cytosol, and the increase in absorbance a t 340 nm due to the formation of CDNBGSH conjugates was recorded for 5 min. Ethoxyresorufin O-dealkylase (EROD) and methoxyresorufin O-dealkylase (MRO D) activities were determined by a fluorometric measurement of resorufin formation as described[10]. The reaction mixtures consisted of 3 mL-50 mmol/L-Tirs buffer (pH 7.5), 25 mmol/L-MgCl2, 1.7 μmol/L-ethoxy-resorufin or 5 μmol/L methoxyresorufin and 1 mg microsomal protein in a quartz cuvette at 20 °C. The reactions were initiated by adding 125 μmol/L NADPH to the cuvette. The increase of fluorescence (excitation wavelength 522 nm and emission wavelength 586 nm) due to resorufin was recorded for 5 min. Enzyme activities were calculated by comparison with a resorufin standard curve. The background activity observed in the absence of microsomal protein was subtracted from each value.
The results were analyzed using a one-way ANOVA procedure, and pair-wise compa risons were made using the Student’s t test procedure in SigmaStat statistical computer software (Jandel Scientific, San Rafael, CA, United States).
The body weight gain of rats over a 10-day period was similar in groups A, B an d C, with the values (g) being 42 g ± 2.8 g, 46 g ± 6.2 g and 45 g ± 5.0 g, respectively (P > 0.05). This suggests that feeding rats with the diet containing 20% Chinese cabbage did not alter their nutrit ional status.
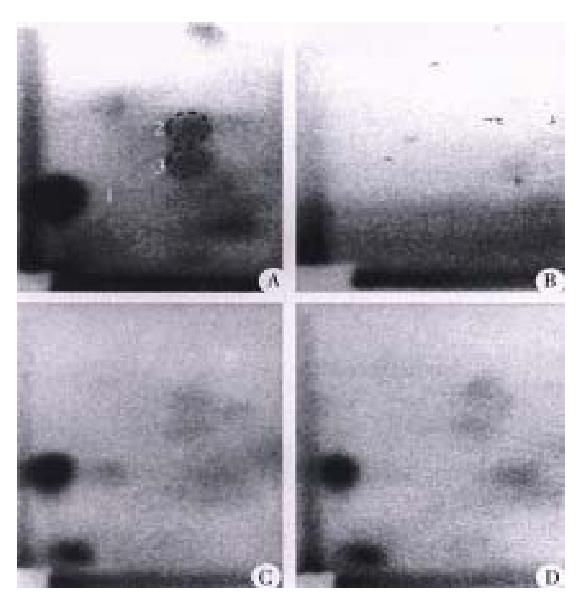
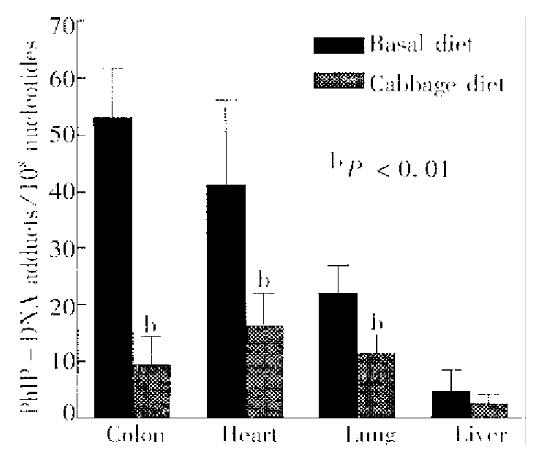
PhIP-DNA adducts were detected by 32P-postlabeling in the heart, lung, liver and colon of rats given PhIP. Adduct pattern was similar in these four tissues and chromatographically identical to that observed in DNA modified in vitro with N-hydroxy-PhIP (Figure 1A, 1C and 1D). The major adduct (spot 1) was previously characterized as N-(2’deoxyguanosin-8-y1)-PhIP[4]. No adducts were detectable in any of the tissues in the control rats without PhIP treatment (Figure 1B). As previously reported[11], DNA adduct levels (adducts/108 nucleotides) were highest in the colon (53.1 ± 8.7), followed in the heart (41.4 ± 14.9) and lung (22.1 ± 5.0), while adduct levels in the liver were relatively low (4.7 ± 3.7) (Figure 2). Following a single dose of PhIP, rats pre-treated with Chinese cabbage had significantly re duced adduct levels in the colon (9.4 ± 5.1), heart (16.3 ± 5.8) and lung (11.4 ± 3.2) as compared with those consuming basal diet without cabbage (P < 0.01). The adduct level in the liver of rats fed cabbage also decreased (2.4 ± 1.7), although the difference from that of control rats was not signifi cant (Figure 2).
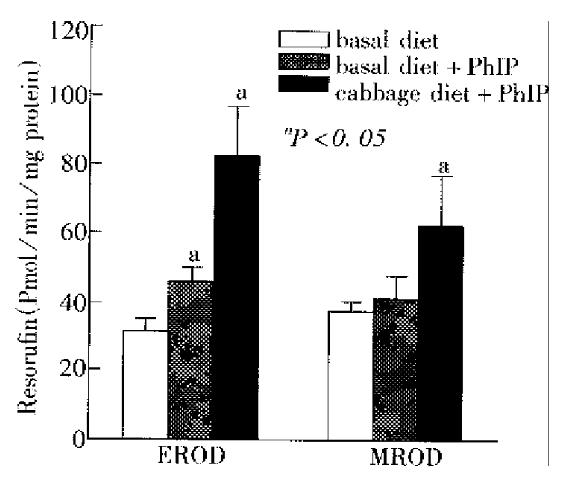
Figure 3 presents results from the EROD and MROD assays. Cytochrome P450 (CYP) 1A1 catalyzes primarily the dealkylation of ethoxyresorufin in the EROD assay, whereas CYP1A2 is mainly responsible for dealkylation of methoxyresorufin in the MROD assay[10]. In rats treated with Chinese cabbage and given a single dose of PhIP, hepatic microsomes displayed a marked induction of EROD activity (82.7 pmol/mg ± 14.1 pmol/mg protein/min). This value was 2.7- and 1.8-fold higher than that in the two control groups (31.1 pmol/mg ± 3.8 pmol/mg and 45.8 pmol/mg ± 3.9 pmol/mg protein/min), respectively (P < 0. 05). Similar results were found in the hepatic MROD assay (Figure 3). In our experiment, rats given basal diet and a single dose of PhIP had EROD activity that was 1.5-fold higher than that of rats given no PhIP, while the MROD activity was not significantly induced as compared with the controls (Figure 3). These results are generally in accorda nce with previous studies showing induction of CYP1A by heterocyclic amines[11].
A comparison of GST activity towards CDNB in the liver, lung and colon mucosa of rats given different treatments indicated that rats pretreated with cabbage had elevated GST activity in the liver and lung. The mean values of the activity inthe liver and lung increased by 18.2% and 35.6%, respectively, as compared with that in controls (P < 0.05). However, no significant differences in GST activity towards CDNB was observed in colon mucosa of rats given different treatments (Table 1).
The present study has shown for the first time that Chinese cabbage inhibits the formation of DNA adducts induced by PhIP. Since carcinogen-DNA adduct formation is generally believed to represent an important genotoxic step in the initiation of carcino-genesis, these results support that Chinese cabbage has a chemoprev entive role against PhIP-induced colon carcinogenesis. The findings are in accordance with epidemiological evidence, which has consistently indicated that regular consumption of brassica vegetables reduces the incidence of human colorectal cancer[8]. Furthermore, our findings provide an insight into the mechan isms of the anticarcinogenic action of vegetables.
PhIP, like most chemical carcinogens, under goes enzymatic biot ransformation in vivo. These metabolic pathways are binary in terms of toxicological consequences, leading to both activation and/or detoxification of the carcinogen. Hydroxylation of exocyclic amino groups of PhIP by CYP1A2 is believed to be an initial activation step, whereas ring-hydroxylation catalyzed principally by CYP1A1 seems to serve as a detoxification pathway[12]. The balance between m etabolic activation and detoxification is one of the important determinants in chemical toxicity and carcinogenicity. We thus examined the induction of CYP1A1 versus 1A2 by Chinese cabbage. It was found that both CYP1A1 and 1A2 were induced by the cabbage, but the induction was preferential for CYP1A1 over CYP1A2 (80.6% vs 50.2%). These results are consistent with a protective effect against PhIP-DNA adduct formation, suggesting that the action mechanism of the cabbage is likely to be via induction of CYP1A1.
Another detoxification pathway of PhIP is GST catalyzed redox reaction of N-acetoxy-PhIP, an ultimate DNA-damaging metabolite of PhIP in vivo, with GSH[13]. The results presented herein clearly demonstrated that Chinese cabbage could induce GST activity in rats. This mechanism may also play an important role in suppression of PhIP-DNA formation in rats pretreated with the cabbage. The oxidation of PhIP by CYP1A2 results in the formation of N-hydroxy-PhIP, which can undergo further activation via O-acetylation by acetyltransferase to form N-acetoxy-PhIP. This metabolic process occurs mostly in the liver and is believed to be an essential step in forming highly reactive electrophiles that cause DNA damage and consequently initiate carcinogenesis. It has been shown that both N-hydroxy-PhIP and N-acetoxy-PhIP can be transported from the liver through the circulation to extrahepatic tissues where it forms PhIP-DNA adducts[14]. Therefore, to protect against PhIP, induction of GST activity in the liver is extremely important because it can eliminate N-acetoxy-PhIP formed in situ and prevent this ultimate carcinogen from being transported to the target tissues. This detoxification pathway may partially explain why the levels of PhIP-DNA adducts in the colon of rats treated with Chinese cabbage were greatly deceased although the activity of GST in the tissue was not induced.
Brassica vegetables are rich in glucosinolates, which can be hydrolyzed to isothiocyanates or indoles. Isothiocyanates and indole derivatives such as indole-3-carbinol are potent inducers of biotransformation enzymes including CYP1A and GSTs[7]. It was shown that the levels of glucosinolates in Chinese cabbage ranged from 170 mg/kg to 1360 mg/kg[7]. The induction of CYP1A and GSTs in rats by Chinese cabbage observed in the present study is lik ely associated with these compounds. Although CYP1A1 mainly catalyzes detoxification of PhIP, it may also catalyze activation of certain carcinogens such as polycyclic aromatic hydrocarbons (PAHs). In view of this fact, one may raise a question that consumption of Chinese cabbage could enhance the carcinogenicity of PAHs, which also widely occurs in the environment. However, many experimental studies have indicated that brassica vegetables and certain hydrolysis products of their glucosinolates also have anticarcinogenic properties against PAHs[7], suggesting that brassica vegetables may preferentially induce enzymes involving detoxification of PAHs. In addition, brassica vegetables also contain many other compounds, which may act-via-different protective mechanisms against chemical carcinogenesis.
In conclusion, this study indicates a preventive effect of Chinese cabbage against a colon carcinogen, PhIP, initiated carcinogenesis in rats, and the mechanism is likely to involve induction of detoxification enzymes. In view of this observation and epidemiological evidence, regular consumption of Chinese cabbage and other brassicas in diet is highly recommended for reducing potential cancer risk of PhIP and other heterocyclic amine carcinogens.
Edited by Xian-Lin Wang
| 1. | Ji BT, Devesa SS, Chow WH, Jin F, Gao YT. Colorectal cancer incidence trends by subsite in urban Shanghai, 1972-1994. Cancer Epidemiol Biomarkers Prev. 1998;7:661-666. [PubMed] |
| 2. | Ito N, Hasegawa R, Sano M, Tamano S, Esumi H, Takayama S, Sugimura T. A new colon and mammary carcinogen in cooked food, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP). Carcinogenesis. 1991;12:1503-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 388] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Boobis AR, Lynch AM, Murray S, de la Torre R, Solans A, Farré M, Segura J, Gooderham NJ, Davies DS. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994;54:89-94. [PubMed] |
| 4. | Lin D, Kaderlik KR, Turesky RJ, Miller DW, Lay JO, Kadlubar FF. Identification of N-(Deoxyguanosin-8-yl)-2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine as the major adduct formed by the food-borne carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, with DNA. Chem Res Toxicol. 1992;5:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Friesen MD, Kaderlik K, Lin D, Garren L, Bartsch H, Lang NP, Kadlubar FF. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5- b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling.. Chem Res Toxicol. 1994;7:733-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition and Prevention of Cancer: a global perspective. Washington, DC, USA: American Institute for Can-cer Research 1997; 216-251. |
| 7. | Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact. 1997;103:79-129. [PubMed] |
| 8. | Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733-748. [PubMed] |
| 9. | Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130-7139. [PubMed] |
| 10. | Sohn OS, Surace A, Fiala ES, Richie JP, Colosimo S, Zang E, Weisburger JH. Effects of green and black tea on hepatic xenobiotic metabolizing systems in the male F344 rat. Xenobiotica. 1994;24:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Degawa M, Kobayashi K, Miura S, Arai H, Esumi H, Sugimura T, Hashimoto Y. Species difference among experimental rodents in induction of P450IA family enzymes by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Jpn J Cancer Res. 1992;83:1047-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Lin DX, Lang NP, Kadlubar FF. Species differences in the biotransformation of the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by hepatic microsomes and cytosols from humans, rats, and mice. Drug Metab Dispos. 1995;23:518-524. [PubMed] |
| 13. | Lin D, Meyer DJ, Ketterer B, Lang NP, Kadlubar FF. Effects of human and rat glutathione S-transferases on the covalent DNA binding of the N-acetoxy derivatives of heterocyclic amine carcinogens in vitro: a possible mechanism of organ specificity in their carcinogenesis. Cancer Res. 1994;54:4920-4926. [PubMed] |
| 14. | Kaderlik KR, Minchin RF, Mulder GJ, Ilett KF, Daugaard-Jenson M, Teitel CH, Kadlubar FF. Metabolic activation pathway for the formation of DNA adducts of the carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rat extrahepatic tissues. Carcinogenesis. 1994;15:1703-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 3.2] [Reference Citation Analysis (0)] |