Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.125
Revised: January 16, 1999
Accepted: January 26, 1999
Published online: April 15, 1999
AIM To study the inducible expression of hepatitis C virus ns3 gene (HCV-ns3) in eukaryotic cells.
METHODS The ns3 gene was obtained from plasmid pBns3 by polymerase chain reaction and inserted into the cloning vector pGEM-T. Then, the ns3 was subcloned into the vector pMSG to generate dexamethasone (DM)inducible expression plasmid pMSG-ns3. CHO cells were transfected by pMSG-ns3 using calcium phosphate precipitation method and cultivated for 12 h-24 h. The transfected cells were induced with DM and the transient expression of NS3 protein was analyzed by ELISA and Western-blot methods.
RESULTS After treated with 3 × 10-8 mol/L DM, the expression of NS3 was observed in the transfected CHO cells. A slightly higher level of NS3 was shown along with the time of DM treatment.
CONCLUSION The inducible expressing vector pMSG-ns3 might be helpful for further studies of the characteristics of the ns3 gene in vivo.
- Citation: Zhang SZ, Liang JJ, Qi ZT, Hu YP. Cloning of the non-structural gene 3 of hepatitis C virus and its inducible expression in cultured cells. World J Gastroenterol 1999; 5(2): 125-127
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.125
Hepatitis C virus (HCV) is a recently identified enveloped positive-strand RNA virus, with a genome size of approximate 9.5 kb, that exhibits a considerable degree of sequence variation. HCV genotypes vary in different geographical areas[1,2]. Data show that the viral protease plays a key role in the HCV polyproteins processing[3-5]. Whether the NS3 protein, a viral protease, has a putative influence during the HCV pathogenesis is unknown. By using PCR method, we obtained the ns3 fragment from the recombinant cloning vect or pBns3 which contained the Chinese HCV ns3 gene and then subcloned it to construct the inducible expressing vector pMSG-ns3. The successful expression of ns3 gene in vitro is helpful for the study of the NS3 protein biological activities in vivo.
Plasmids Recombinant vector pBns3 was provided by the Microbio logical Department; cloning vector pGEM-T vector system kit was purchased from Promega; and expressing vector pMSG was provided by Dr. Wen-Chao Zheng.
CellsE. Coli DH5α and CHO cells were preserved in our department.
PCR primers design and synthesis Primers were provided by Dr. Su-Ming Wang (Human Gene Therapy Research Institute). Postive primer: 5’-GTCGCTAGCCATGGAATTCCCTACG-3’ with the Nhe I (GCTAGC) restriction site, the initation codon ATG and the Kozak sequence. Negative primer:5’-CGGCGCTCGAGTGGAATTTCATACAA-3’ with the Xho I restriction site (CTCGAG). The recombinant plasmids pBns3 were used as template to amplify the ns3-690 bp fragments.
Main reagents Restriction enzymes, T4 ligase, calf intestinal alkaline phosphatase (CIP), protoblot Western-blot AP system kit and fmol DNA sequencing system kit were all purchased from Promega, QIA quick gene gel kit from QIA gene. Human anti-HCV serum and anti-HCV EIA kit (TMB) were provided by the Microbiology Department.
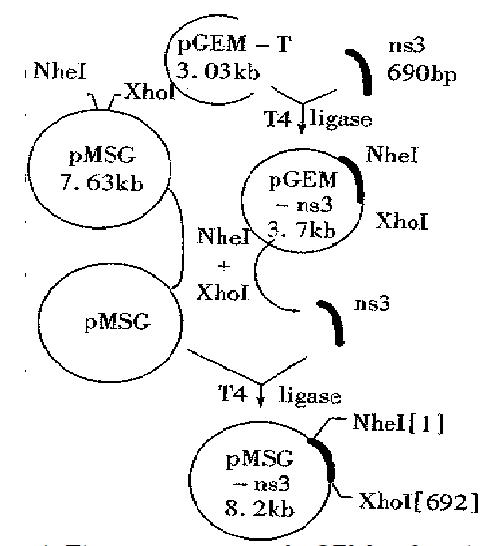
Plasmid construction The 690 bp fragments of ns3 gene were amplified from the recombinant plasmid pBns3 by PCR and subcloned into the cloning vector pGEM-T. The positive clones selected from the transfected DH5α were performed as described by the pGEM-T vector systems (Promega). The constructed plasmids (designated as pGEM-ns3) were identified by the restri ction enzyme analysis and verified by sequencing. The corresponding ns3 gene was excised from the pGEM-ns3 digested with Nhe I and Xho I and then religated with the pMSG to generate the dexamethasone (DM)inducible expressing plasmids pMSG-ns3 (Figure 1).
Transient expression of NS3 protein was analyzed in CHO cells transfected with the pMSG-ns3 by the calcium phosphate coprecipitation method. 4 h after the addition of DNA, the cells were glycerol shocked with 10% glycerol in PBS for 2 min and replenished with fresh medium to grow for 24 h. After 8 h, 24 h and 32 h in the presence of DM, respectively, the transfected cells were collected to detect the NS3 proteins. The similar cell preparations untransfected by the pMSG-ns3 served as negative control. The collected cells were treated with lysis buffer (50 mM-Tris.cl, pH 8.0, 10 mg/L- phenyl methylsufonyl fluoride, 1% Nonidet P-40) for 20 min in ice bath and then centifuged for 15 min in a microfuge . The supernatants were isolated and prepared to be analyzed by ELISA and Western-blot methods.
Results were shown as -x±s (mean ± SD) and a paired Student’s t test was used for quantitative information, and P < 0.05 was considered statistically significant. Statistical analysis of the results was carried out using Duncan’s multiple range test with computer.
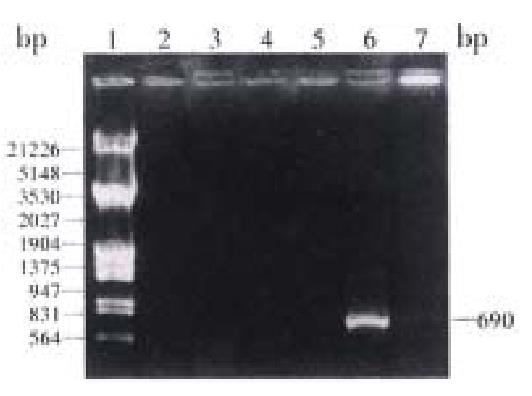
Amplification of HCV-ns3 by PCR Using the plasmid pBns3 as template, PCR was performed to amplify a 690 bp fragment and then cloned into Xho-I and Nhe-I sites of the cloning vector pGEM-T (Figure 2).
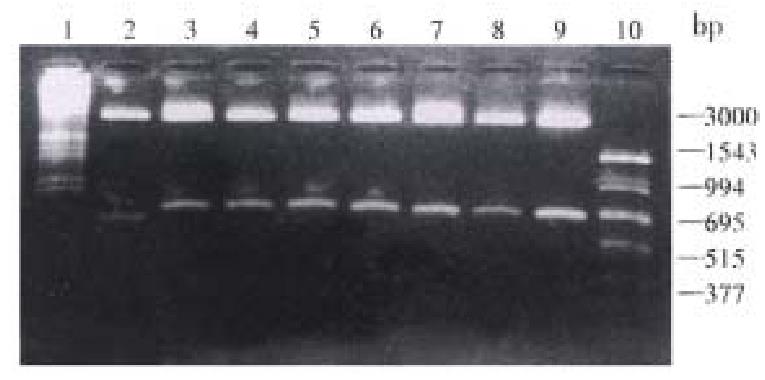
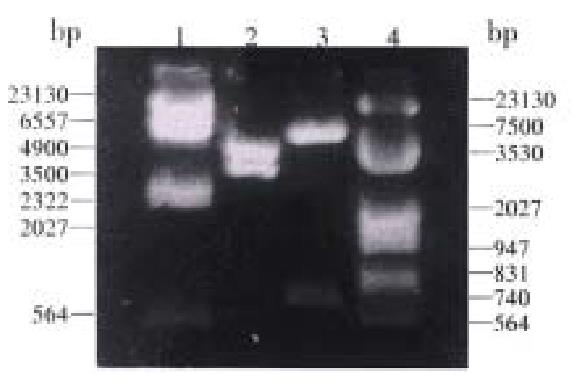
Plasmid construction All plasmids described in this study were constructed in DH5-α. The extracted DNA sequencing showed that the inserted fragments contained: the Kozak sequence, the initiation codon ATG and the Nhe I, Xho I restriction sites; the 690 bp corresponding sequence of the ns3 gene; the correct reading frame of the NS3 proteins (Figures 3 and 4).
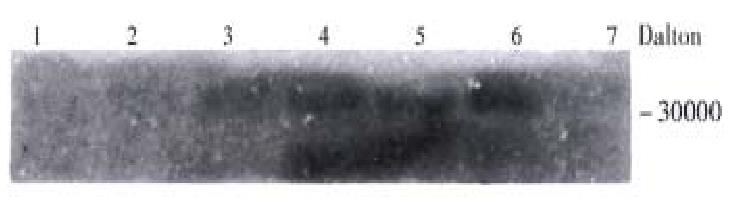
Expression of the NS3 proteins in mammalian cells In the groups transfected by DM induced pMSG-ns3, the ELISA results were different as compared with the negative control (P < 0.05) (Table 1). A-M-r 30000 protein was detected by Western blot analysis (Figure 5).
| Serial dilution | Negative control | Treatment periods (t/h) | |||
| 0 | 8 | 24 | 32 | ||
| 1:16 | 0.08 ± 0.02 | 0.18 ± 0.01a | 0.20 ± 0.01a | 0.20 ± 0.02a | 0.22 ± 0.01a |
| 1:32 | 0.04 ± 0.02 | 0.19 ± 0.02a | 0.17 ± 0.01a | 0.18 ± 0.01a | 0.22 ± 0.01a |
| 1:64 | 0.02 ± 0.00 | 0.14 ± 0.02a | 0.15 ± 0.01a | 0.14 ± 0.01a | 0.20 ± 0.02a |
| 1:128 | 0.03 ± 0.02 | 0.12 ± 0.01a | 0.15 ± 0.00a | 0.12 ± 0.01a | 0.16 ± 0.01a |
| 1:256 | 0.01 ± 0.01 | 0.06 ± 0.01a | 0.09 ± 0.03a | 0.11 ± 0.01a | 0.07 ± 0.01a |
Epidemiological data show that more than 1% of the world population are infected with HCV and HCV patients often develop chronic hepatitis, with long-term c omplications of cirrhosis and hepatocellular carcinoma (HCC)[6]. Some reports suggest that several viral proteases do harm their hosts, such as human immunodeficiency virus type 1 protease (HIV-1 PR) which can destroy the cytoske letons (the latter involves the cell shapes, the initiation of mitoses may play an essential part in cell signal transportions) and cause the cell abnormal proliferation leading to carcinogenesis[7,8]. Whether the NS3 proteins, also being a viral protease, would injure the host cytoskeletons and attribute to the high incidence of HCC in HCV patients is worth investigation.
To facilitate the expression of NS3 protein in vitro in our study, an inducible expressing vector pMSG-ns3 containing both the initiation codon ATG and the Kozak sequence (GCCATGG) was created. Surprisingly, however, no significant differences of ELISA results were found in other cells (such as Hela, SMMC-7721, etc.). Under the low level of the protein expressio n, the non-specific agents existing in the cell extracts from the human beings might have across-reaction with the detection antibody to cause a high background. The same initiation codon might be affected by several regulation elements existing in different cells and lead to different efficiencies in the express ion. To avoid the interferences resulting from certain non-specific agents, CHO cells were used as transfected hosts. Subsequently, the NS3 proteins with Mr-30 000 were detected in all the transfected groups after the treatment of DM and their contents increased slightly along with the time of treatment. Although the ELISA results of the transfected group apparenly increased in the absence of DM (P < 0.05), no NS3 proteins were detected by the Western-blot analysis. Thus, in order to eliminate the fault results, the Western-blot method are more reliable and convincible for analyzing the proteins expression than the other means such as ELISA, etc.. Our study of the transient expres sion of NS3 proteins in vitro is not only available for further selecting cell lines with a stable integrants of ns3 gene, but also helpful for the development of the ns3 transgenic mice and the study of the NS3 biological funct ions in vivo.
Project supported by the National Natural Science Foundation ofChina, No.39470290.
Edited by Xian-Lin Wang
| 1. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4657] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 2. | Qi ZT, Pan W, Kong XT. Immunoscreening and identification of Chinese HCV genomic cDNA gt11 Library. Acad J Sec Mil Med Univ. 1992;13:401-410. |
| 3. | Tomei L, Failla C, Santolini E, De Francesco R, La Monica N. NS3 is a serine protease required for processing of hepatitis C virus polyprotein. J Virol. 1993;67:4017-4026. [PubMed] |
| 4. | Takamizawa A, Mori C, Fuke I, Manabe S, Murakami S, Fujita J, Onishi E, Andoh T, Yoshida I, Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991;65:1105-1113. [PubMed] |
| 5. | Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H. Nonstructural protein 3 of the hepatitis C virus encodes a serine-type proteinase required for cleavage at the NS3/4 and NS4/5 junctions. J Virol. 1993;67:3835-3844. [PubMed] |
| 6. | Plagemann PG. Hepatitis C virus. Arch Virol. 1991;120:165-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Shoeman RL, Höner B, Mothes E, Traub P. Potential role of the viral protease in human immunodeficiency virus type 1 associated pathogenesis. Med Hypotheses. 1992;37:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Wang Ning. Modulation of cytoskeletal stiffness and cancer inhi-bition In: LI Chun-Hai, GUO Ya-Jun, eds.Current Advance in Tu-mor Molecular Biology. Beijing: Military Medical Science Press. 1996;53-56. |