Published online Apr 15, 1999. doi: 10.3748/wjg.v5.i2.103
Revised: March 18, 1999
Accepted: March 27, 1999
Published online: April 15, 1999
AIM To determine whether proliferating cell nuclear antigen (PCNA) is present in the peripheral circulation and whether PCNA levels correlate with enhanced regenerative activity.
METHODS In animal studies, adult male Sprague-Dawley rats (n = 3-4/group) were sacrificed at 0, 12, 24, 36, 48, 72 and 96 h following 70% partial hepatectomy. At each interval, sera were analyzed by Western blot for PCNA by two monoclonal antibodies (PC-10 and 19F-4). In human studies, sera from 4 patients with liver cirrhosis and 4 healthy controls were tested in a similar manner.
RESULTS The PC-10 monoclonal antibody identified a protein with a molecular mass of 120 KD which remained stable in rat sera for 24 h fol lowing partial hepatectomy, then increased 1.5-fold at 48 h prior to retur ning to baseline at 96 h after partial hepatectomy. However, it was not dete cted in the sera of patients with or without liver disease. In the 19F-4 monoclonal antibody, a protein with a molecular mass of approximately 46 KD was found. which was present in rat sera prior to partial hepatectomy and for 12 h after surgery. Thereafter, levels fell by approximately 50% at 24 h, 65% at 36 h and 75% at 48 h where they remained until 96 h after partial hepat ectomy. The decrease in levels correlated with the extent of partial hepatectomy. In human sera, the appearance of this inhibitory cell nuclear antigen (ICNA) was higher in the sera of patients with cirrhosis than in healthy controls.
CONCLUSION The PC-10 monoclonal antibody can detect a protein in the circulation when active hepatic regenerative activity is taking place. The 19F-4 monoclonal antibody, however, identifies a protein in both rat and human sera that inversely correlates with hepatic regenerative activity. This prote in which is tentatively referred to as inhibitory cell nuclear antigen (ICNA) may be used in documenting the extent of suppression of hepatic regeneration.
- Citation: Assy N, Gong Y, Zhang M, Minuk G. Appearance of an inhibitory cell nuclear antigen in rat and human serum during variable degrees of hepatic regenerative activity. World J Gastroenterol 1999; 5(2): 103-106
- URL: https://www.wjgnet.com/1007-9327/full/v5/i2/103.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i2.103
Serologic markers of hepatic regenerative activity are lacking. Those that are available (ornithine decarboxylase, thymidine kinase and alpha-fetoprotein levels) correlate with enhanced regenerative activity and only appear when the stimulus to regeneration is significant (large partial hepatectomies)[1]. To date, serologic markers of attenuated hepatic regenerative activity has yet been identified. In this study, we set out to determine whether proliferating cell nu clear antigen (PCNA) might serve as a more sensitive marker of enhanced hepatic regenerative activity than presently available enzymatic assays.
PCNA is a 261 amino acid nuclear protein that has been identified as an auxiliary protein of DNA polymerase delta and is involved in the progression of cell cycle and DNA synthesis [2,3]. Monoclonal antibodies to PCNA are increasingly used for evaluation of cell proliferation in situ[4,5]. Of the 17 known monoclonal antibodies to PCNA, PC-10 and 19F-4 are two of the most commonly used ones[6,7]. Recently, using the PC-10 and the 19F-4 monoclonal antibodies, we demonstrated that tissue levels of PCNA correlate well with hepatic regenerative activity when compared with PCNA immunostaining, [3H] thymidine incorporation into DNA and other markers of hepatic regenerative activity[8].
In the present study, we employed both the PC-10 and 19F-4 monoclonal antibodi es in an attempt to determine whether circulating PCNA levels correlate with regenerative activity in rats following partial hepatectomy and in humans with various forms of liver diseases.
Adult male Sprague-Dawley rats (250 g/body-300 g/body weight) were mainta ined on Purina rat chow and water ad libitum until the day prior to surgery. All animals were kept in similar housing units on a 12 h light and 12 h dary cycle. Thirty and 70% partial hepatectomies were performed under light ether anesthesia between 9:00 a.m. and noon each day according to the methods of Hi ggins and Anderson[9]. Sham operations in which appropriate portions of the liver were exteriorized for the same length of time as rats undergoing partial hepatectomy were also carried out. Blood samples (1 mL-2 mL) were taken from groups of rats ( n = 3-4/ group) at the time of death by exsanguination at 0, 12, 24, 48, 72 and 96 h after surgery. Sera from the samples were stored at -20 °C until batch tested. Sera were also collected from 4 patients with histologic evidence of liver cirrhosis (2 HBV, and 2 HCV related) and from 4 healthy laboratory volunteers.
Equal amounts of serum protein (100 μg/lane) as quantified by the Lowry method[10], were passed through a 10% SDS-PAGE at 100 V for 2 h at room temperature. Resolved proteins were transferred electrophoretically to nitrocellulose (Bio-Rad, Hercules, CA) at room temperature for 1 h at 100 V in a buffer containing 25 mM-glycine, 192 mM Tris, and 20% m ethanol. Nonspecific binding of the antibodies to the membranes was dim inished by preincubating the blots in TBS (50 mM-Tris-HCl and 150 mM-NaCl, pH 7.4) in the presence of 3% dry milk for 1 h at room temperature. Blots were incubated with PC10 monoclonal antibody (1:500, DAKO Corporation, Carpintena, CA) and 19F4 monoclonal antibody (1:500, Boehringer Mannheim, Germany) in TBS containing 0.5% dry milk and 0.1% Tween-20 ( Bio-Rad, Hercules, CA) at 4 °C for 16 h followed by peroxidase-labeled ant i-mouse antibody (1:500 dilution, Amersham, Quebec) in the same buffer at room temperature for 1 h. After each incubation, blots were washed twice with TBS containing 0. 1% Tween-20 for 10 min. Immunoreactive bands were visualized using an enhanced chemiluminescence kit (ECL, Amersham, Quebec). Films were scanned by a PC scanner and the optical densities (OD) determined by an NIH. IMAGE program (NIH, Bethesday, Maryland).
Data were presented as means ± SD. Differences between groups and differences over time were analyzed using the Man-Whitney test, repeated measure analysis of variance and Kruskal-Wallis analysis where appropriate. P values less than 0.05 were considered significant.
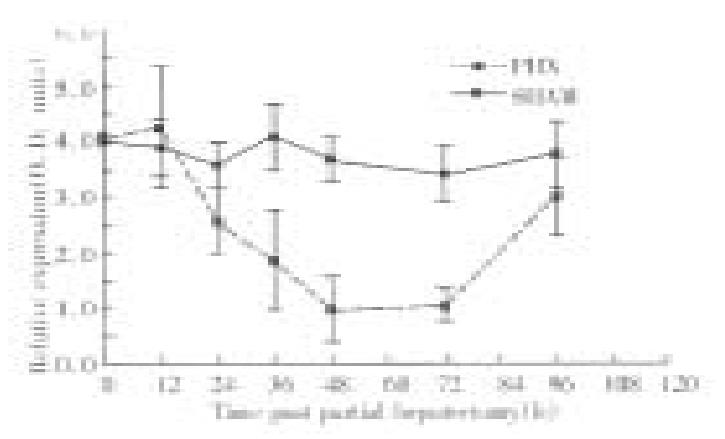
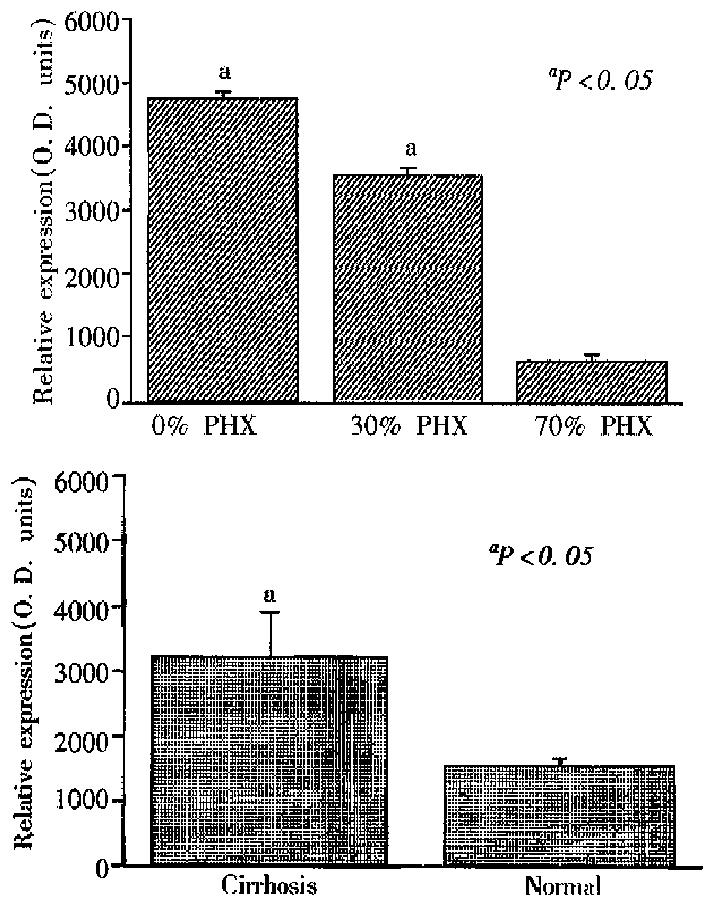
Figure 1 provides the results of 19F-4 protein determinations in rat sera follo wing partial hepatectomy. As reported elsewhere, the molecular mass of the 19F-4 protein was 46KD. In sham operated rats, 19F-4 levels remained unchanged throughout the study. However, in partial hepatectomized rats, 19F-4 levels fell significantly to a nadir of approximately 50% baseline values at 24 h after partial hepatectomy (P < 0.001). Thereafter, levels remained low until 96 h when they returned to baseline. Figure 2A shows the results of serum 19F-4 levels following various degrees of partial hepatectomy at 48 h after surgery. Levels were lowest in rats having undergone 70% partial hepatectomy, highest in sham operated controls and intermediate in the 30% partial hepatectomy group (P < 0.05). Finally, in the human sera, 19F-4 was again identified at 46KB, but levels were significantly higher in cirrhotic patients than in healthy controls (Figure 2B, P < 0.05).
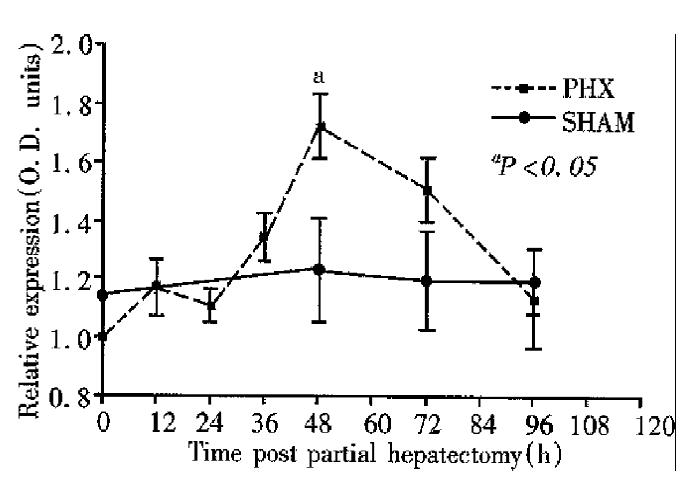
The results of sera PC-10 protein determinations are shown in Figure 3. The molecular mass of PC-10 was 120KD. Serum levels remained relatively constant for 24 h prior to a gradual increase of approximately 1.5 times baseline at 48 h following partial hepatectomy (P < 0.05). Serum levels of PC-10 were not detected in either cirrhotic patients or healthy controls.
Two proteins were described in rat sera that crossreact with monoclonal antibodies to proliferating cell nuclear antigens. The results also indicate that the levels of these crossreacting proteins changes significantly following partial hepatectomy and in the case of 19F4.4, they change inversely in proportion to the extent of partial hepatectomy. They are also higher in sera of cirrhotic patients as compared with healthy controls.
The cross reactivity reported here does not represent an isolated phenomenon with such antibodies. Waseem et al[6] reported that PC-10 reacted with a faint band at 120KD besides reacting with 36KD protein HeLa cell. However, no investigation of the functional role of this crossreacting protein was given in a model of liver regeneration, neither was this protein documented in the blood previously. The fact that PC-10 protein increased significantly at 48 h after partial hepatectomy stresses its possible role as a marker of liver regeneration in the blood. Whether the crossreactivity is due to direct binding to the immunogenic peptide of new proteins or involve secondary changes of the PCNA mole cule (degradation product) with difference in the amino acid sequences of the im munogenic protein is unknown.
A similar crossreactivity has been shown with other monoclonal antibodies especially when used with different concentrations[11-13]. However, the concentration of the monoclonal antibodies used here were the same as used previously in the liver tissues and in which no crossreacting proteins were seen[8]. Thus, differences in concentration are unlikely responsible for the crossreactivity observed here. Another possibility is that the crossreactivity is due to the binding of the secondary antibody (anti-mouse IgG) with the protein, however incubation of the membrane with secondary antibody alone did not reveal the above crossreacting protein suggesting that the crossreactivity is due to the presence of monoclonal antibodies.
It must be appreciated that identical or closely related similar epitopes may occur on otherwise unrelated protein[11]. The 19F-4 protein expression shown here may reflect a profile of an inhibitory protein during liver regeneration.
Three explanations are in favor of the above hypothesis. First, it decreased sharply to reach a nadir level at 24-36 h after partial hepatectomy, a period that corresponded to the peak of DNA synthesis[14]. Second, the 19F-4 protein was expressed more in the sera of patients with cirrhosis than in the sera of normal persons. Finally, and most importantly, the fact that 19F-4 protein expression decreased significantly with the extent of hepatic resection may reflect its possible role as an inhibitory protein which is synthesized by the liver.
It has been established that the 19F-4 and PC-10 reacts with a protein region of 14 at length[15]. The reasons why both monoclonal antibodies detect the same PCNA protein at a molecular mass of 36KD in the liver tissue while each detect or immunoreact with different molecular mass protein in the blood, is unknown (PC-10-120KD and 19F-4-46KD). Theoretically, the unsophisticated epitopes recognized by these monoclonal antibodies might be present in another protein in the blood. One explanation for the inability of PC-10 to recognize the peptide recognized by 19F-4 in the blood is the length of the protein. In a recent mini-review, Laver et al[16] stressed that conformational epitopes on native proteins are comprised of 15-22aa residues.
One could suggest that the early appearance of 19F-4 protein followed by a decrease at 24 h after partial hepatectomy reflect a parameter of injury or represent an acute phase reactant protein rather than being related to the process of growth after partial hepatectomy. However, the appearance of the protein in t he serum of sham rats argues against this possibility, moreover, the presence of such a protein in the serum of normal persons, also argue against this hypothesis.
PC-10 crossreacting protein (120KD) correlates positively with the regenerative rate after 70% partial hepatectomy whereas 19F-4 protein correlates negatively. The 19F-4 (46KD) protein is clearly of interest in that it is a cell cycle dependent protein, being undetectable in the serum during rapidly dividing cells, prominent in the serum of intact normal liver and more prominent in the serum of patients with liver cirrhosis. Crossreacting proteins detected by monoclonal antibodies are not always non-specific and fortuitous but may have an important role in clinical biology. Ongoing studies to isolate and sequence the corresponding protein are under investigation.
Edited by Jing-Yun Ma
| 1. | Fausto N. Hepatic regeneration. In: D Zakim, TD Boyer, eds. Hepatology: a textbook of liver disease. Philadelphia: W.B. Saunders. 1990;49-62. |
| 2. | Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987;326:517-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 825] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 3. | Jaskulski D, deRiel JK, Mercer WE, Calabretta B, Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988;240:1544-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 317] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Yang CL, Zhang SJ, Toomey NL, Palmer TN, Lee MY. Induction of DNA polymerase activities in the regenerating rat liver. Biochemistry. 1991;30:7534-7541. [PubMed] |
| 5. | Theocharis SE, Skopelitou AS, Margeli AP, Pavlaki KJ, Kittas C. Proliferating cell nuclear antigen (PCNA) expression in regenerating rat liver after partial hepatectomy. Dig Dis Sci. 1994;39:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Waseem NH, Lane DP. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990;96:121-129. [PubMed] |
| 7. | Ogata K, Kurki P, Celis JE, Nakamura RM, Tan EM. Monoclonal antibodies to a nuclear protein (PCNA/cyclin) associated with DNA replication. Exp Cell Res. 1987;168:475-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 178] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Assy N, Gong Y, Zhang M, Pettigrew NM, Pashniak D, Minuk GY. Use of proliferating cell nuclear antigen as a marker of liver regeneration after partial hepatectomy in rats. J Lab Clin Med. 1998;131:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Higgins GM, Anderson RM. Experimental pathology of the liver: Restoration of the liver of the white rat following partial surgical removal. Arch Path. 1931;12:186-201. |
| 10. | LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 11. | Nigg EA, Walter G, Singer SJ. On the nature of crossreactions observed with antibodies directed to defined epitopes. Proc Natl Acad Sci USA. 1982;79:5939-5943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Dulbecco R, Unger M, Bologna M, Battifora H, Syka P, Okada S. Cross-reactivity between Thy-1 and a component of intermediate filaments demonstrated using a monoclonal antibody. Nature. 1981;292:772-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Lane DP, Hoeffler WK. SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature. 1980;288:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | GRISHAM JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842-849. [PubMed] |
| 15. | Roos G, Landberg G, Huff JP, Houghten R, Takasaki Y, Tan EM. Analysis of the epitopes of proliferating cell nuclear antigen recognized by monoclonal antibodies. Lab Invest. 1993;68:204-210. [PubMed] |
| 16. | Laver WG, Air GM, Webster RG, Smith-Gill SJ. Epitopes on protein antigens: misconceptions and realities. Cell. 1990;61:553-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 408] [Article Influence: 11.7] [Reference Citation Analysis (0)] |