Published online Feb 15, 1999. doi: 10.3748/wjg.v5.i1.28
Revised: September 19, 1998
Accepted: October 26, 1998
Published online: February 15, 1999
AIM To study the pathogenesis of hepatotoxicity of halothane.
METHODS The effect of different concentration of halothane and sevoflurane on mitochondrial membrane phospholipids composition of rat liver were analyzed using high performance liquid chromatography (HPLC) technology.
RESULTS Halothane at low concentration could degrade mitochondr ial membrane major phos-pholipids and increase lysophosphatidylcholine.
CONCLUSION The pathogenesis of halothane hepatotoxicity was the phospholipids variation on liver mitochondria.
- Citation: Sui B, Zhang GM, Yu WF, Wang XM, Ma YD, Liu SX. Experimental research on phospholipids variation of halothane on liver mitochondria. World J Gastroenterol 1999; 5(1): 28-30
- URL: https://www.wjgnet.com/1007-9327/full/v5/i1/28.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i1.28
The effect of traditionally inhalational anesthetic halothane and new drug sevof lurane on mitochon-drial membrane is reported below in an attempt to study the pathogenesis of halothane hepatotoxicity.
According to modified Estabook’s velocity gradient method[1], the mitochondria of male rat weighing 150 g-200 g was separated. Seventy mmol sucrose and 220 mmol bovine serum albumin were used as isolation medium. Albumin was assayed by biuret reaction. The mitochondria concentration was ad-justed to 10 g/L-30 g/L. Phospholipids except for ganglioside and acetal phospholipid were extracted using improved Higgins’ method[2]. The mito chon-drial suspension was mixed well with the extraction solvent (1:10, V/V ), and stood for 15 min. The albumin was removed by centrifugation. CaCl2 0.05 mL/L was added to the supernatant, and stood for centrifugation (3000 r/min). The lower layer was evaporated to dryness under nitrogen at 40 °C-50 °C. After added with diluent accurately to the residue, the solution was sealed to protect from light and stored at -20 °C for HPLC analysis. The whole procedure was carried out at 4 °C in the air-tight ice-bath.
The standard control phospholipids of phos-phatidylethanolamine (PE), phosphatidy linositol (PI), phosphatidylserine (PS), phosphatidylcholine (PC), cardiolipin (CL), sphingomyclin (SPH) and lysophosphatidylcholine ( LPC ) were purchased from Sigma Co.
Extracting solution: chloroform:methanol:hy-drochloric acid (2:1:0.01,V/V/V).
Moving phase: n-hexane:isopropanol:ethanol: potassium dihydrogen phosphate (25 mnol/L) : glacial acetic acid (370 : 485 : 100 : 562 : 0.1 V/V/V/V). The solution was evenly mixed and stood overnight for separating phosphoric acid crys-tal. After ultrafiltration and deoxygenation the su-pernatant was used as moving phase.
Standard solution: the standard control phos-pholipids were dissolved in the mixture of n-hexane: isopropanol (6 : 8, V/V). The concentration was 2 g/L.
ISUZU LC-6A liquid chromatograph, ISUZU Shim-Pack CLC-SIC column (6 mm × 15 cm), Guarc PAKTM prepared column were used. The detection was performed at 206nm. After reaction of low concentration and high concentration of halothane or sevoflurane with mitochondrial membrane phos-pholipids, 10 mL reaction solution was taken out for repeated injection[3]. Each sample was repeated for 8 times, and linear velocity (mm/min) was recorded. Qualitative analysis was made by identification of the retention time with standard control samples. The eluting sequence referred to Patton sequence[4]. Quantitative ana lysis was made by calculating the peak area and the relative content of phospholipids was expressed by the ratio between peak area and albumin.
By comparing the HPLC chromatograph peak of phospholipids affected by halothane at low and high concentration with that of the normal liver mito-chondria phospholipids, it could be seen that the main phospholipid peak decreased to some degree and LPC peak increased, especially when at high concentration. Sevoflurane at low concentration had no influence on phospholipid peak, but at high concentration it could decrease the main phospho-lipid peak and increase the LPC peak. However, the effect was not so obvious as that caused by halothane.
The change of liver mitochondrial phospholipids caused by halothane and sevoflur ance is shown in Table 1. Halothane at both high and low concentration could decrease the main liver mitochondrial phospholipids and increase LPC significantly. The change of phospholipids had no significant difference between sevofurane at low concentration and the control while at high concentration the difference was marked. At high concentration, the change of phospholipids in liver mitochondria-caused by halothane was much more obvious than that caused by sevoflurane.
| Group | PE | PI | PS | CL | PC | SPH | LPC |
| Control | 1.48 ± 0.26 | 1.16 ± 0.19 | 0.84 ± 0.09 | 1.02 ± 0.11 | 2.93 ± 0.28 | 3.98 ± 0.59 | 4.54 ± 0.42 |
| At low concentration | |||||||
| Sevoflurane | 1.37 ± 0.19 | 1.08 ± 0.09 | 0.78 ± 0.07 | 0.95 ± 0.11 | 2.72 ± 0.17 | 3.76 ± 0.33 | 4.89 ± 0.33 |
| (-7.62%) | (-7.12%) | (-6.93%) | (-6.55%) | (-7.19%) | (-5.52%) | (+7.81%) | |
| Halothane | 1.19 ± 0.12 | 0.96 ± 0.06 | 0.70 ± 0.80 | 0.85 ± 0.16 | 2.50 ± 0.26 | 3.37 ± 0.29 | 5.90 ± 0.12 |
| (19.60%)ab | (-17.24%)ab | (-16.94%)ab | (-16.25%) | (-14.38%)ab | (15.43%)ab | (+30.1%)ab | |
| At high concentration | |||||||
| Sevoflurane | 1.30 ± 0.12a | 1.02 ± 0.04a | 0.63 ± 0.04a | 0.89 ± 0.70a | 0.64 ± 0.16a | 3.57 ± 0.22a | 5.69 ± 0.32c |
| (11.89%) | (12.02%) | (-11.26%) | (-12.55%) | (-10.36%) | (-10.40%) | (+25.35%) | |
| Halothane | 1.06 ± 0.09c | 0.81 ± 0.07c | 0.78 ± 0.09c | 0.75 ± 0.09c | 2.09 ± 0.16c | 2.95 ± 0.24c | 7.81 ± 0.67c |
| (-28.38%)b | (-2.41%)b | (-25.24%)b | (-26.59%)b | (-2867%)b | (-26.61%)b | (+41.87%)b |
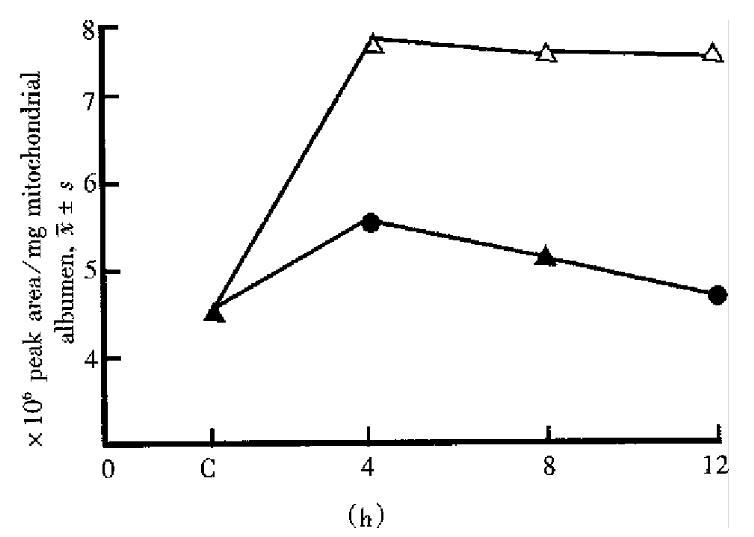
The effect on the liver mitochondrial phospholipid principle started and went up rapidly as soon as halothane contacted with mitochondria and reached the peak at 4 h. At low concentration it could recover to the level of the control group at 6 h-8 h while at high concentration it could not even within 24 h. In each phase there had no significant difference between low concentration of sevoflurane and the control. At high concentration the effect caused by sevoflurane reached the peak at 4 h and recovered to the control level at 8 h-12 h. The time-phase change on LPC by halothane and sevoflurane at high concentration is demonstrated in Figure 1.
This study indicated that halothane at low concentration could degrade mitochondrial membrane major phospholipids and increase LPC, at high concentration it could damage mitochondrial membrane ir-reversibly. Although sevothane had action on mito-chondria, the effect was reversible. Probably due to its molecular stru cture halothane soluble in liver mi-tochondria easily and destroy phospholipids obviously. Halothane had the similar result in the study on the inhalational anesthetic effect on liver mitochon-dria intimal fluidity[5].
Phospholipase A (PLA1, PLA2) is universal in liver membrane. Characterized by intramembranous mode of action, PLA2 has a high activity in mito-chondria and the highest catalytic speed toward PE (twice that of PC, ten times of CL). PLA2 could be excited by Ca equilibration of liver cell caused by poison in vivo and in vitro. The phospholipid struc-ture variation greatly influences biomembrane func-tion and physical property including membrane congugase and receptor kinetics[6]. Some other stud-ies showed that lipid variation such as mitochondrial phospholipids degradation and lipid peroxidation is an important original cause of liver cell damage. Destruction of the integration of mitochondria is the result of mutual function of the above-mentioned two mechanisms while degradation of membrane phospholipid caused by activation of mitochondria probably plays a more important role in the early damage of overall function of liver cells[7].
Besides hypoxia and low volume of blood flow the study also showed that mitochondrial phospho-lipids variation in the unorganized test is the main factor of halothane hepatotoxicity. Inhabition of PLA2 activity and antilipid peroxidation may be the important measure of antihalothane hepatotoxici-ty[8].
Edited by Jing-Yun Ma
| 1. | Estabrook IH. Oxidative and phosphorylation. Methods in enzymology. Vol 10. New York: Academic Press Inc. 1997;45; 10003-10012. |
| 2. | Higgins JA. Separation analysis of membrane lipid components, In: Findlay JBC, ed: Biological membrane: a practical approach. Washington DC: IRC Press. 1987;103-107. |
| 3. | Yu WF, Liao MY. Effect of sevoflurane, anflurane, isoflurane and halothane on mice isolated liver mitochondrial respiration function. Chin J Anesthesiol. 1986;16:121-123. |
| 4. | Patton GM, Fasulo JM, Robins SJ. Separation of phospholipids and individual molecular species of phospholipids by high-performance liquid chromatography. J Lipid Res. 1982;23:190-196. [PubMed] |
| 5. | Sui B, Yu WF, Liao MY. The effect of inhalational anesthetic on fluxion property of liver mitochondria. Chin J Anesthesiol. 1995;15:561-562. |
| 6. | Liu MS, Kang GF, Ghosh S. Activation of phospholipases A1 and A2 in heart, liver, and blood during endotoxin shock. J Surg Res. 1988;45:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Yeagle PL. Lipid regulation of cell membrane structure and function. FASEB J. 1989;3:1833-1842. [PubMed] |
| 8. | Yu WF, Wang JY, Liu SY. The hepatotoxicity of halothane and sevoflurane on primary culture mice liver cell. Chin J Anesthesiol. 1993;13:243-246. |