Published online Feb 15, 1999. doi: 10.3748/wjg.v5.i1.12
Revised: November 29, 1998
Accepted: January 4, 1999
Published online: February 15, 1999
AIM To investigate the expression of perforin and fas-ligand (fas-L) of tumor infiltrating lym-phocytes (TILs) in human hepatocellular carci-noma (HCC).
METHODS By in situ hybridization and immuno-histochemistry, the perforin and fas-L gene ex-pression of TILs was studied in 20 HCC cases. RESULTS Positive expression of perforin and fas-L genes was detected in 16 HCC cases. One patient had expression of perforin and fas-L genes in the majority of TILs and survived 1.5 years after tumor resection without HCC re-lapse. This seems that the presence of a large number of activated T cells might be beneficial for the antitumor immunity. In other cases, less than 10% o f TILs were able to express perforin and fas-L genes.
CONCLUSION Although there were a number of T cells in HCC, only few of them were im-munoactive and able to kill tumor cells. It seems important to promote further proliferation of these activated T cells in vitro or in vivo.
-
Citation: Qian QJ, Xue HB, Qu ZQ, Fang SG, Cao HF, Wu MC.
In situ detection of tumor infiltrating lymphocytes expressing perforin and fas ligand genes in human HCC. World J Gastroenterol 1999; 5(1): 12-14 - URL: https://www.wjgnet.com/1007-9327/full/v5/i1/12.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i1.12
Cytotoxic T lymphocytes (CTLs) play a major role in killing tumor cells. Two pat hways have been described by which a cytotoxic cell may induce lysis of its target[1,2]. The first pathway is called perforin pathway. T cell receptors (TCRs) of CTLs binding with MHC antigens on the tumor cells induce the release of granules filled with perforins and granzymes, and perforins then attack the target cell surface, followed by granzymes entering into the cell and killing it. The second is fas-L pathway. Fas-L from activated T cell binds with Fas antigen on the tumor cell surface, directly causing cell apop-tosis. Although we applied TILs to treat tumor sev-eral years ago[3], this is the first domestic report studying whether activated T cells around the tumor express the two killing genes, perforin gene and fas-L gene. We studied the expression of perforin and fas-L genes of TILs in the HCC specimens using in situ hybridization and immunohistochemistry to find out whether there are T cells with killing activities in HCC tissues.
Specimens were obtained from 20 HCC patients (16 men and 4 women; ranging in age from 25 to 64 years with a mean of 47 years) who underwent tu-mor resection from January to June, 1994 in our hospital. Among these patients, 17 were associated with liver cirrhosis. The control specimens were from the normal liver tissues of 3 patients with hep-atic angioma. The normal liver tissues and HCC tis-sues were quickly frozen in liquid nitrogen within half an hour after removed from the bodies of the patients, and stored at -70 °C. The specimens cut from the marg in between tumor and paratumor areas were embedded with O. C. T, fixed with 40 mL/L-paraformaldehyde, gradiently dehydrated with ethanol, and then stored at -70 °C. Human fas-L cDNA was kindly presented as a gift by Dr. Nagata of Japanese Bioscience Institute, and human perforin cDNA by Dr. Kevin Y.T. Thia of Aus-tralia Austin Institute. Rabbit-anti-human fas-L polyclonal antibodies were purchased from Santa Cruz Biotech Company of USA. Rat-anti-human perforin monoclonal antibody was donated by Dr. Eckhard. R. Padack of the Medical Academy of Miami University, USA. Digoxin labeling and detection kit was purchased from Boehringer Mannheim Company of Germany. The immunohis-tochemistry kit of streptavi din-biotin amplification system was a-product of WAK Company of Ger many.
HE staining
In situ hybridization Probe labeling proceeded according to the method of random primer labeling presented by the digoxin labeling kit of Boehringer Mannheim Company. In situ-hybridization was performed following a previously published protocol[3]. The concentration of the probe was 1 × 103μg/L and cells with blue granules under microscope were regarded as positive.
Immunohistochemistry The specimens were processed according to the ABC method, and visualized with DAB. Cells with brown granules under micro-scope were regarded as positive.
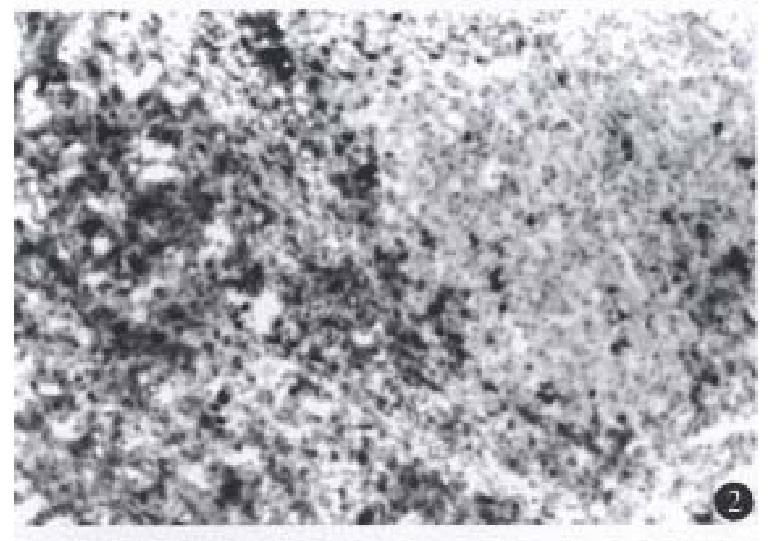
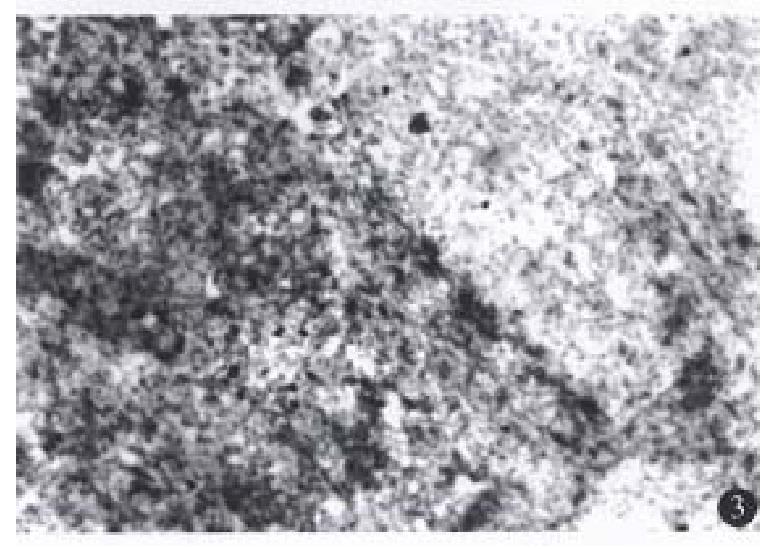
A few lymphocytes with negative perforin and fas-L expression were seen in the 3 normal liver tissues, while in the 19 HCC specimens, there was a various number of TILs, most in the mesenchyma of the tu-mor and a few in the parenchyma. Furt hermore, in another HCC case (No.14) which had not-expe-rienced relapse for 1.5 years after tumor resection, there were a large number of TILs with positive expression of perforin and fas-L not only in the mesenchyma of the tumor, but also extensively in the parenchyma of the liver (Figures 1, 2 and 3). TILs of 15 patients had positive perforin and fas-L expres-sion with a positive rate below 10%. In the other four cases, although there was a various number of TILs infiltrating in the tumor mesenchyma, no TILs expressed perforin and fas-L. There was no relationship between the number of TILs in the liver tissue and the positive rate of perforin and fas-L expression.
There were no perforin and fas-L positive-hybri-dization signals in the 3 normal liver specimens. In HCC specimens, perforin and fas-L expression of TILs showed strong positivity in one case (No.14), mild positivity in 15 cases and negativity in 4 cases, indicating that perforin and fas-L expression in transcriptive level was parallel to that in protein level in HCC.
TILs, a heterogeneous group of lymphocytes con-sisting largely of T lymphocytes, are located mainly in the tumor mesenchyma. As early as 1907, TILs were found in tumor tissues and the phenomenon that lymphocytes infiltrated in tumor tissues was supposed to be the result of the resistance of the host to the tumor. Later, it was found that the greater the number of TILs in the tumor tissue, the better outcome the patient would get[4].
Early studies on TILs focused on their pheno-types. Recent investigations showed that T lympho-cytes killed the tumor cells mainly under the per-forin and fas-L pathways. Perforin and fas-L ex-pression in TILs of tumor tissues is directly related to the killing activity of TILs in tumor tissues. In 1994, Leger-Ravet et al[5] found perforin and granzyme B expression in TILs in 10 follicular lymphoma cases. Of all 20 HCC cases we studied, TILs expressed perforin a nd fas-L in varing degrees in 16 patients, and negatively in 4. This indicates that cytotoxic T lymphocytes existed in most of the HCC patients. Furthermore, in one case there was a large number of TILs expressing fas-L and perforin in both the mesenchyma and the parenchyma of the tu-mor tissues. This patient did not sustain relapse within the 1.5 year follow-up period. This implies that large quantities of T lymphocytes with killing activities existing in the tumor tissues are beneficial for the prognosis of HCC patients. Except the case presen ted above, TILs expressing perforin or fas-L in the other 19 cases of HCC were below 10%, showing that most of the TILs were in an immuno-suppressive state. The mechanism of this phe-nomenon might be (1) the HCC could not activate T lymphocytes due to its deficient expression of the second signal B7; (2) T lymphocytes expressing high levels of fas and fas-L resulted in self-apoptosis[6,7]. Therefore, amplification of the T lymphocytes with killing activities in vivo or in vitro may improve the therapeutic effect for the patients with tumors.
Edited by Jing-Yun Ma
| 1. | Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1161] [Cited by in RCA: 1179] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 2. | Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 796] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 3. | Guo YJ, Wu MC, Kong XT. Immuno-response in primary hepatocellular carcino-ma and paratumor tissues. Chin J Exp Sur. 1987;4:545-547. |
| 4. | Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1992;29A:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Leger-Ravet MB, Devergne O, Peuchmaur M, Solal-Celigny P, Brousse N, Gaulard P, Galanaud P, Emilie D. In situ detection of activated cytotoxic cells in follicular lymphomas. Am J Pathol. 1994;144:492-499. [PubMed] |
| 6. | Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95). Nature. 1995;373:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1278] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 7. | Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 679] [Cited by in RCA: 708] [Article Influence: 23.6] [Reference Citation Analysis (0)] |