Published online Dec 15, 1998. doi: 10.3748/wjg.v4.i6.540
Revised: September 25, 1998
Accepted: November 14, 1998
Published online: December 15, 1998
AIM: To study the expression of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells in order to analyze the possible relationship between cell growth regulation by alpha-fetoprotein(AFP) and Fas/Bcl-2 proteins.
METHODS: BEL-7404 human hepatoma cells were maintained in RPMI 1640 medium supplemented with 10% new-born calf serum. Cells adhered to coverslips were used to detect Fas and Bcl-2 protein expression by the avidin-biotin complex (ABC) immunocytochemical assay.
RESULTS: Immunocytochemical study showed that essentially all the BEL-7404 human hepatoma cells could express Fas and Bcl-2 proteins, although in various amount. No positive staining for Fas and Bcl-2 proteins was observed when cells were incubated with non-relevant sera, to establish the specificity.
CONCLUSION: Fas apoptosis signals and Bcl-2 rescue/survival signals from apoptosis are expressed in BEL-7404 human hepatoma cells. The finding strongly implys that AFP-mediated cell apoptosis and growth enhancement are potentially associated with Fas and Bcl-2 proteins present in those cells.
- Citation: Wang XW, Xie H. Presence of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells. World J Gastroenterol 1998; 4(6): 540-543
- URL: https://www.wjgnet.com/1007-9327/full/v4/i6/540.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i6.540
Alpha-fetoprotein (AFP) is a major serum protein present in the early stages of development in mammals and other vertebrates, which virtually disappears in adult life. This protein is mainly synthesized by the fetal liver and the yolk sac. But, AFP may reappear in some tumors such as human primary hepatocellular carcinoma. It has also been found that AFP, like other serum proteins, can bind to specific membrane receptors on some cells such as macrophages, T lymphocytes, hepatoma cells, breast carcinoma cells and so on. AFP has immunoregulatory functions in a variety of experimental systems and some experimentally induced autoimmune diseases in animals can also be inhibited by the administration of AFP[1]. Our recent studies demonstrate that human AFP stimulates the proliferation of hepatoma cells in vitro[2]. Further investigation found that high concentrations of AFP resulted in hepatoma cell growth arrest[3], which suggests that AFP may be a biphasic cell growth regulator. However, the mechanisms of the growth-regulatory properties exhibited by AFP are largely unstudied.
Apoptosis routinely occurs during embryogenesis, histogenesis, metamorphosis, endocrine-dependent tissue atrophy and normal adult tissue turnover. Moreover, tumor regression is also often mediated through apoptosis as a result of X-irradiation and chemotherapeutic exposure in cancer cells[4,5]. The detection of apoptotic cells and apoptotic bodies is based on several well-established morphological features. These features include cell shrinkage, disconnection with neighboring cells, nuclear chromatin condensation, maintenance of cytoplasmic membrane integrity, strong eosinophilic cytoplasm and lack of an inflammatory reaction. Fragmentation of the cells leads to the appearance of membrane-bound apoptotic bodies. Apoptotic bodies are defined as small, roughly spherical or ovoid cytoplasmic fragments, some of which contain nuclear fragments. A previous report demonstrated that AFP and AFP-receptor antibody blocked the induction of apoptosis in HL-60 leukemia cells in culture[6]. But, high concentrations of AFP was found to induce apoptosis in human hepatoma cells[7]. These results have strong implications that growth-regulatory activity of AFP is, at least in part, related to affecting apoptosis precess.
It has now been ascertained that cell apoptosis signals and rescue (survival) signals from apoptosis are mediated by a cell-surface transmembrane protein termed Fas and a cytosplasmic protein termed Bcl-2[8-10]. The human Fas protein (also designated APO-1) is a 48 kDa cell surface glycoprotein that belongs to a family of receptors that includes CD 40, nerve growth factor receptors and tumor necrosis factor receptors. A series of studies indicate that apoptosis is mediated by the intercellular interactions of Fas with its ligand (Fas-L) or effectors. On the other hand, bcl-2 has been identified as an apoptosis inhibitor. The Bcl-2 protein (molecular mass 25 kDa) is encoded by a gene involved in the t (14, 18) chromosomal translocation and plays a central role in the inhibition of apoptosis. On the basis of Genbank identification, an amino acid sequence resembling a Fas-like peptide stretch has been detected in human AFP (39% identity, 23 amino acids in length). In a similar fashion, a sequence identifying with a Bcl-2-like amino acid stretch can also be discerned in human AFP ( 41% identity, 17 amino acids in length)[11]. Therefore, we speculate that Fas and Bcl-2 proteins in human hepatoma cells may contribute to the influence of AFP on apoptosis and growth regulation. We hereby investigated the expression of Fas and Bcl-2 proteins in BEL-7404 human hepatoma cells with avidin-biotin complex (ABC) immunocytochemical method.
A human hepatoma cell line, BEL-7404, was maintained in RPMI 1640 medium (Gibco) supplemented with 10% new-born calf serum, at 37 °C, 5% CO2 and 100% humidity. The RPMI 1640 medium was replaced with fresh medium every two to three days. For ABC assy, cells at a densitty of 5 × 103 cells/mL were grown on coverslips, and adhensive cells were directly used. Cell viability was determined by mixing the cell suspension with 0.5% trypan blue (1:1).
Antibodies used included a rabbit polyclonal antibody to Fas protein (Santa Cruz), and a mouse monclonal antibody to Bcl-2 protein (Dako). As described by the manusfacturers, the antibody against Fas protein is an affinity-purified antibody raised against a peptide corresponding to amino acids 260-279 mapping at the carboxy terminus of Fas protein of human origin. The specificity of the antiserum for Fas was confirmed by Western blotting and immunohistochemistry. The antibody against Bcl-2 protein belonged to mouse IgG1 sub-class. The antibody reacted specifically with Bcl-2 protein as demonstrated by immunoblotting and immunoprecipitation.
The monoclonal and polyclonal antibody reactivities were visualized using Vectastain ABC kit (Vector) following the vendor’s instructions with a few modifications[12]. Briefly, adhensive cells were fixed in 4% paraformaldehyde for 5 min-8 min at room temperature, then treated with 0.05 M Tris-buffered saline (TBS, pH7.2) containing 0.4% Triton X-100 for 15 min at room temperature. The coverslips were then incubated with 1% bovine serum albumin in TBS containing 0.4% Triton X-100 for 30 min at 37 °C. For the detection of Fas protein, prior to incubation with antibodies, endogenous peroxidase activity was irreversily inhibited by treatment with 5% hydrogen peroxide in methanol solution at room temperature for 30 min. After washing in TBS, primary antibodies to Fas were applied for 1 h at 37 °C. After washing 3 times, coverslips were incubated with biotinylated sheep anti-rabbit secondary antibodies at 37 °C for 45 min followed by avidin biotin peroxidase complex at 37 °C for 45 min. For color development, a peroxidase substrate solution, 0.05% 3,3’-diaminobenzidine (Sigma) in TBS containing 0.01% hydrogen peroxide, was applied for 5 min-10 min at 37 °C. For the assay of Bcl-2 protein, primary antibodies for Bcl-2 were reacted with the cells for 1 h at 37 °C. After incubation with the biotinylated secondary antibodies (horse anti-mouse antibodies) for 45 min at 37 °C, the coverslips were treated with alkaline-phosphatase-conjugated avidin-biotin complex for 1 h at 37 °C. Development reagents contained 33 μL of nitroblue tetrazolium salt (75 mg/mL in 70% dimethylformamide) and 25 μL of 5-bromo-4-chloro-3-indocyl phosphate (50 mg/mL in dimethylformamide) in 7.5 mL TBS. For color development, cells were incubated in the color solution for up to 4 h at 37 °C in the dark. The coverslips were finally rinsed in water. The primary antibodies were replaced by control non-relevant sera to monitor specificity of ABC immunocytochemical staining.
On the basis of preliminary experiments, rabbit anti-human Fas antibody was used at a dilution of 1:80, sheep anti-rabbit IgG at 1:50, and rabbit ABC at 1:100, respectively. The dilution of mouse anti-human antibodies against Bcl-2 was 1:100. Horse anti-mouse IgG and mouse ABC were used at 1:80 and 1:100, respectively. The use of appropriately diluted reagents minimized troublesome nonspecific background staining.
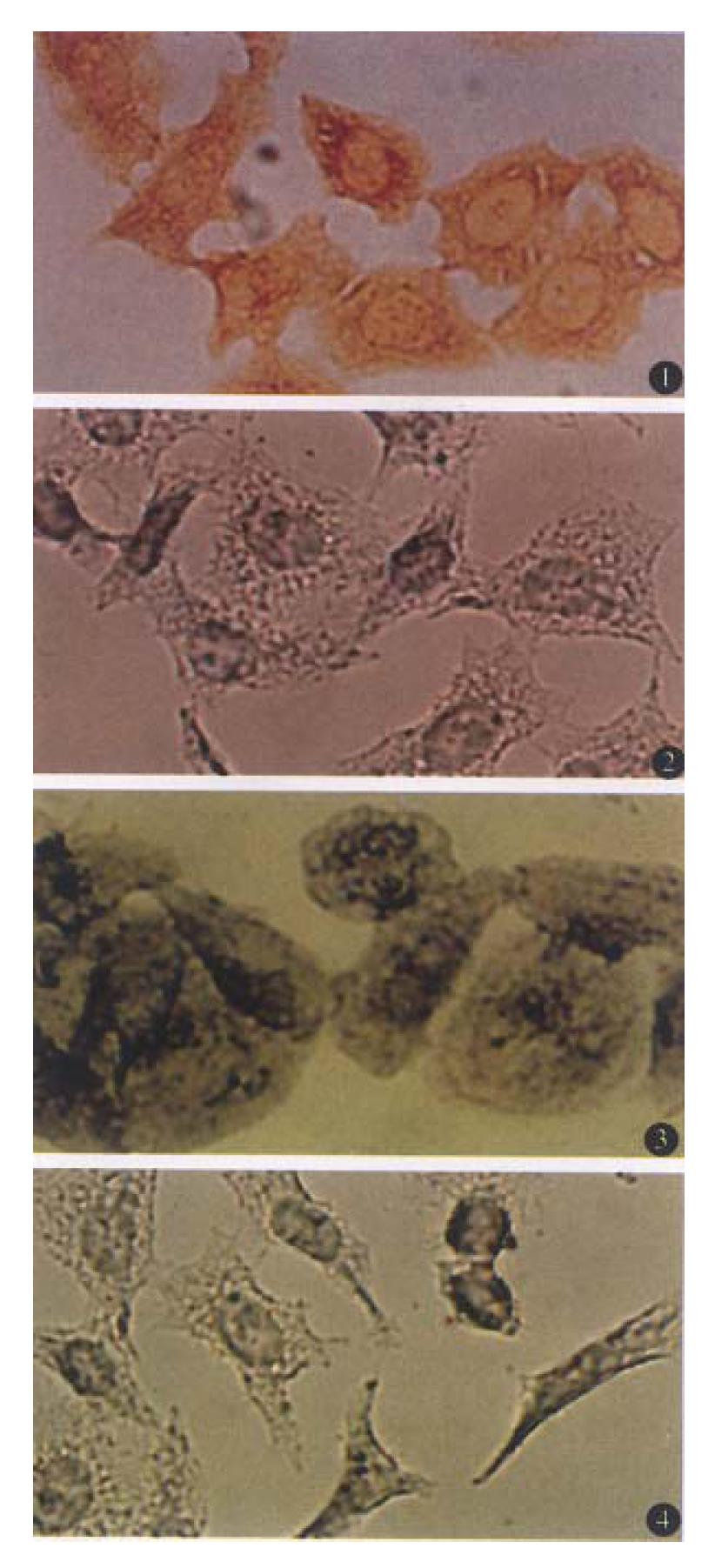
Figure 1 (1) shows ABC detection of Fas antigen in BEL-7404 cells. When primary antibodies to Fas were incubated with BEL-7404 cells, large numbers of brown grains were detected on the membrane of essentially all the cells, although the grain distribution varied. Treatment of the cells with normal rabbit serum resulted in a marked reduction in the number of specific grains (Figure 1 (2)). When the primary antibodies against Bcl-2 were used, purple grains were present diffusely throughout the cytoplasm of BEL-7404 cells with sparing of the nuclei. The number of grains in the cytoplasm varied, but essentially all the cells, including those undergoing mitotic division, were considered to contain Bcl-2 protein (Figure 1 (3)). However, when cells were incubated with normal mouse serum under the same experimental condition, no accumulation of grains in the cells was observed (Figure 1 (4)).
Overdevelopment of the color reaction can produce a high level of non-specific staiing, but this was significantly reduced by careful monitoring of the reaction after the addition of color reagents.
For study of gene expression, immunocytochemical staining can be used to detect final products of gene expression and their location in cell, though it cannot be used to detect directly genes. In the present study, Fas and Bcl-2 proteins have been detected by ABC immunocytochemical method in cultured BEL-7404 human hepatoma cells. We consider that ABC assay is rapid, reliable, sensitive and economical to use, and there are no known health hazards associated with the reagents used, which provides a very useful tool for investigating the gene expression[12].
Some studies demonstrate that cell apoptosis is mediated by the intercellular interactions of Fas with its ligand or effectors, and Bcl-2 protein can inhibit apoptosis[8-10]. As mentioned in the “Introduction”, amino acid sequences of Fas and Bcl-2 proteins can be detected on some domains of human AFP[11]. Moreover, Bcl-2 site is localized adjacent to a proposed hinge region of human AFP molecule, which allows rotational flexibility. A conformational change in the tertiary structure of AFP, possibly induced by excessive ligand binding, can expose such a Bcl-2 site, which is normally hidden in a molecular crevice. According to the present result, taken together with previous published data, we speculate that dimerization or binding of low concentrations of AFP with its normal molecular configuration to Fas protein could blunt the apoptosis signals, resulting in enhancement of cell growth. On the other side, dimerization or binding of high concentrations of AFP with possible conformational change to the exposed Bcl-2 signal site could block the rescue/survival signals, causing the induction of apoptosis. In this fashion, AFP might function in both the up and down-regulation of cell growth by employing a binding or dimerizing mechanism to apoptotic mediators.
The oncoproteins are products of the proto-oncogenes, which now include myc, myb, fos, jun, ski, ets, cbl, erb A and possibly many others. Most oncoproteins have turned out to be transcription factors, which function as molecular switches that sense incoming signals and modulate the transcription of specific genes. Many oncoproteins have functional partners with which they heterodimerize to bind DNA, such as fos and jun, myc and max, etc. Protein-protein and protein-DNA interactions of the oncoproteins are often mediated through helix-loop-helix and leucine zipper motifs. Tumor suppressor proteins (p53 and retinoblastoma protein) can function as growth suppressors. It has been found that AFP gene promoter can be regulated by some transcription factors such as AP-1[13], although the activation of the oncogenes c-fos, c-jun and c-myc might not directly in volve the basal level of AFP gene expression in hepatoma cell lines[14]. The mutation of p53 gene occurred in the early stage of hepatocarcinogenesis may be correlated with the initiation of hepatocarcinogenesis, and mutant P53 protein probably related to the reactivation of AFP gene[15]. On the other hand, Genbank computer-generated sequence identities detected on human AFP domains demonstrated that amino acid identities for the oncogenes c-erb A, c-myc, rel, myb, ras ranged from 29% to 54% over lengths from 13 to 24 amino acid, largely in human AFP domains 1 and 2. In comparison, the tumor-suppressor (retinoblastoma, Rb) protein identities appear to reside only on domain 1 of human AFP[11]. Thus, it is possible that the growth-regulatory activity of AFP may also be mediated by some oncoproteins and/or tumor suppressor proteins. The exact relationship between AFP growth regulation and oncoproteins as well as tumor suppressor proteins still needs to be further investigated in the future.
Project supported by National Postdoctor Science Foundation of China, No. 199711.
| 1. | Wang XW, Xie H. Significance of alpha-fetoprotein in the development of novel therapeutic agents. Drugs Fut. 1998;23:in press. |
| 2. | Wang XW, Xu B. Stimulation of tumor-cell growth by alpha-fetoprotein. Int J Cancer. 1998;75:596-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Dudich E, Semenkova L, Gorbatova E, Dudich I, Khromykh L, Tatulov E, Grechko G, Sukhikh G. Growth-regulative activity of human alpha-fetoprotein for different types of tumor and normal cells. Tumour Biol. 1998;19:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 5. | Marx J. Cell death studies yield cancer clues. Science. 1993;259:760-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Laderoute MP, Pilarski LM. The inhibition of apoptosis by alpha-fetoprotein (AFP) and the role of AFP receptors in anti-cellular senescence. Anticancer Res. 1994;14:2429-2438. [PubMed] |
| 7. | Semenkova LN, Dudich EI, Dudich IV. Induction of apoptosis in human hepatoma cells by alpha-fetoprotein. Tumour Biol. 1997;18:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879-886. [PubMed] |
| 10. | Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1619] [Cited by in RCA: 1686] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 11. | Mizejewski GJ. alpha-fetoprotein as a biologic response modifier: relevance to domain and subdomain structure. Proc Soc Exp Biol Med. 1997;215:333-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10193] [Cited by in RCA: 10429] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 13. | Bois-Joyeux B, Thomassin H, Richard F, Ikonomova R, Denissenko M, Danan JL. [Several transcription factors participate in the functioning of the alpha-fetoprotein gene promoter]. Bull Cancer. 1995;82:541-550. [PubMed] |
| 14. | Tournier-Thurneyssen I, Feldmann G, Bernuau D. Nuclear oncogenes and alpha-fetoprotein gene expression in hepatoma cell lines. Tumour Biol. 1993;14:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Zou HQ, Tang ZY, Ye SL. [Mutation of p53 gene and expression of alphafetoprotein during hepatocarcinogenesis]. Zhonghua Yixue Zazhi. 1994;74:474-475, 474-475. [PubMed] |