Published online Oct 15, 1998. doi: 10.3748/wjg.v4.i5.459
Revised: June 9, 1998
Accepted: August 26, 1998
Published online: October 15, 1998
- Citation: Yi XR, Kong XP, Zhang YJ, Tong MH, Yang LP, Li RB. High expression of human augmenter of liver regeneration in E. coli. World J Gastroenterol 1998; 4(5): 459-460
- URL: https://www.wjgnet.com/1007-9327/full/v4/i5/459.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i5.459
Heat-stable hepatocyte stimulatory activity has been described in the liver of weanling rats and pigs. This growth factor is called hepatocyte stimulator substance (HSS)[1]. Hagiya et al[2] reported the complete amino acid sequence of a 30KDa band from their purified product of rat liver and the cloning and sequence analysis of its cDNA. They called it augmenter of liver regeneration (ALR). Our previous study[3] demonstrated that human ALR had been cloned and sequenced. To further study the bioactivity of human ALR (hALR), we constructed a highly expressed vector pBV-hALR in E. coli and about 20% of somatic protein of rhALR was expressed.
Enzymes such as EcoRI, BamHI and Hind III were purchased from Promega. pBV220 plasmid and E. coli JM109 were stored and pGEM-hALR plasmid was constructed in our laboratory[3].
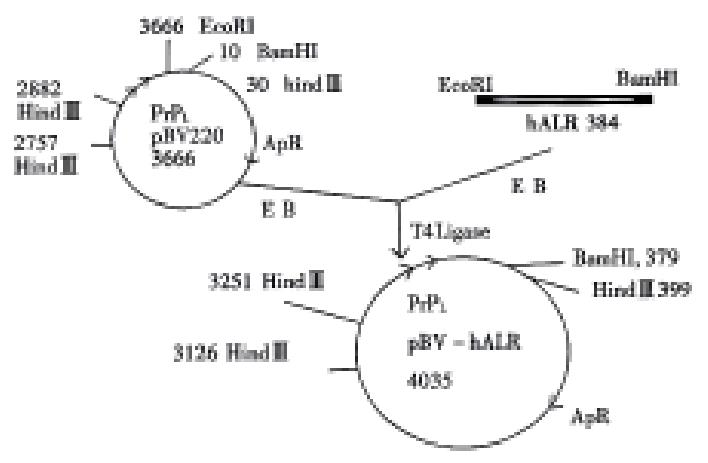
hALR cDNA fragment was obtained from low-melting gel after electrophoresis of pGEM-hALR plasmid DNA which was digested with EcoRI and BamHI. pBV220 vector DNA was also digested with EcoRI and then incubated at 75 °C for 10 min. hALR cDNA fragment with compatible cohesive termini and pBV220 DNA fragment were connected in bacteriophage T4 DNA ligation system at 14 °C overnight. The reaction contained T4 DNA ligase 1 μL (3u), buffer 10 × 1 μL, 120 μg hALR cDNA fragment and 100 μg pBV200 DNA fragment. Five μL of ligation mixtures was added to 200 μL comptent E. coli JM109. Transfer appropriate volume of transformed cell onto LB plate agar containing ampicillin (100 mg/L) at 37 °C overnight. Bacterial colonies that contain hALR plasmids by digestion were identified on plasmid DNA with restriction enzymes (EcoRI, BamHI) and agarose gel electrophoresis.
LB medium containing ampicillin (100 μg/mL) was inoculated with one colony which contain hALR plasmid to 0. D600 = 0.4-0.5 at 30 °C and the temperature of the culture was regulated to 42 °C, and the incubation was continued for 5 h.
It refers to reference[4].
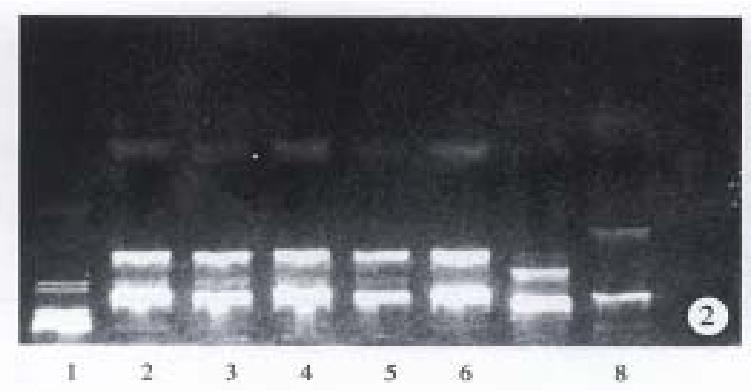
hALR cDNA fragment was inserted into pBV200 vector (Figure 1) and 6 positive colonies were detected and identified by Hind III. pBV200 vector showed 3 fragments 2727 bp, 783 bp and 125 bp and pBV-hALR vector also showed 3 fragments, 2727 bp, 1183 bp and 125 bp, but the second fragment appeared differently (Figure 2).
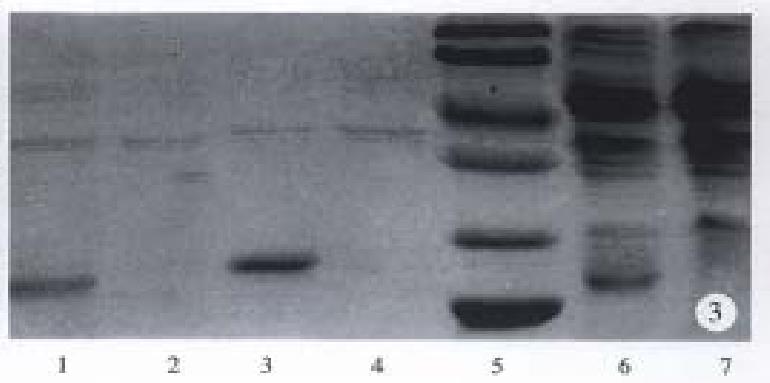
Induced by temperature, the hALR gene was highly expressed in E. coli, and SDS-PAGE showed that 20% of somatic protein of rhALR was expressed (Figure 3). Most of rhALR protein existed as inclusion bodies in E. coli. After isolation and washing the purity of granule reached 70% (Figure 3).
Since LaBreeque[1] reported a kind of heat-stable, liver specific stimulator in weanling liver extraction, the gene has been cloned for about 20 years, but in vain. In 1994 Hagiya[2] found a similar stimulator to HSS (ALR). The recombinant ALR eventually produced by the gene derived from the purified rat cytosol retained the hepatotrophic potency as the native peptide, with no effect on the cultured hepatocytes. ALR is not only expressed in liver tissue but also in thymus. With the availability of the hALR gene and its rhALR product we think a series of questions can be explained about its specificity, heat-stable quality and mechanisms. Its potential clinical implication for the treatment of liver diseases, including fulminant liver failure, is worth further studies.
| 1. | LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 162] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T, Shimonishi M, Porter KA, Starzl TE. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci USA. 1994;91:8142-8146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Yi XR, Tong MH, Kong XP, Yang LP, Li RB, Zhang YJ. Molecular cloning and sequence analysis of rat and human augmenter of liver regeneration. World Chin J Digest In press. . |
| 4. | Lu SD. Current protocols for molecular biology. Beijing: Advanced Education Press. 1993;. |