Published online Oct 15, 1998. doi: 10.3748/wjg.v4.i5.426
Revised: May 13, 1998
Accepted: July 6, 1998
Published online: October 15, 1998
AIM: To investigate whether the arcuate nucleus (ARC) could modulate gastric motility, and if so, what are the mechanisms or pathways.
METHODS: Wistar rats, anaesthetized with urethan, parameters of stimulation and electrolytic lesion sites were determined according to the Paxinos and Watson “ATLAS of rat brain in steriotaxic coordinate”. Intragastric pressure ( IGP ) and gastric motility were measured by Reybould¡äs method.
RESULTS: Electrical stimulation of ARC could obviously decrease the IGP by 42.2% ± 5.4%, n = 15, P < 0.01, and the phasic gastric contractions disappeared. The analysis showed that the locus coeruleus (LC) and dorsal raphe (DR) nuclei may be involved in central, but without the invovement of β-endorphinergic neurons rich in the ARC, while in periphery, the peripheral neural pathways are both vagus and sympathetic nerves. The fibers in vagus may be non-cholinergic. Humoral factors may also be involved. At the receptor level, Tonic action of adrenergic nerve in the stomach is mainly inhibitory; β-receptors, which may be present on the stomach wall and mediate inhibition; and α-receptors, which come into play through vagus, mediate inhibition, but those present on the smooth muscle mediate sympathetic excitation. Microinjection of TRH into ARC could significantly increase the IGP by 183.02% (0.53 kPa ± 0.08 kPa vs 1.5 kPa ± 0.6 kPa, n = 10, P < 0.001), the rate and amplitude of phasic gastric contraction were also increased (3 cpm vs 6 cpm-8 cpm). The peripheral pathway of such excitatory effects were transmitted with cholinergic vagus nerve mediated by M-receptor.
CONCLUSION: ARC could modulate gastric motility biphasically, inhibitory and excitatory, depending on the nature of stimuli.
- Citation: Xu GY, Ma R, Cao Q, Su BT. Modulation of hypothalamic arcuate nucleus on gastric motility in rats. World J Gastroenterol 1998; 4(5): 426-429
- URL: https://www.wjgnet.com/1007-9327/full/v4/i5/426.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i5.426
Arcuate nucleus (ARC) is the third largest nucleus of the hypothalamic nuclei with a volume of 0.94 mm3 in rats. It is located at the base of hypothalamus, and surrounds the ventral part of the third ventricle. At least 15 neurotransmitters and neuropeptides, e.g. β-endorphin, enkephalin, etc. have been found in ARC. The ARC is anatomically organized to communicate primarily with the pituitary gland, hypothalamus, limbic system, certain thalamic nuclei, the midbrain periaqueductal gray and autonomic nuclei of the brainstem. This general organization leads us to hypothesize that it may play a key role in integrating autonomic functions. But so far there has been no report showing that ARC could modulate gastric motility. This study was designed to clarify whether ARC modulates gastric motility, and if so, to analyse their mechanisms or pathways.
Wistar rats, both sexes, weighing 250 g-300 g, fasted 24 h before experiment, but free to have water, were anesthetized with urethan and paralysed with flaxedil.
Intragastric pressure (IGP) and gastric motility were measured according to Reybould’ s method through pressure tranducer (type YH-II, Institute of Space Medicine Enginering), and recorded by two channels physiological pen-recorder (type LSM-2B, Chengdu Instrument Factory). The rate of IGP changes are calculated according to the following equation:
Math 1
Subdiaphragmatic vagotomy was performed according to Mordes (1978) method, and extirpation of celiac neural plexus by Moraes (1978) method. Experiments began 2-3 weeks after operation and sham operation used as control.
Rats were fixed at stereotaxic instrument (type SN-2, Narishige, NIHON, KONDON). The reference coordinates were: for ARC, p 3.5 mm-3.8 mm, H 10.0 mm-10.5 mm, L or R 0.2 mm; and for LC, p 9.5 mm-9.8 mm, H 7.0 mm-7.1 mm, L or R 1. 1 mm-1.3 mm; for DR, p7.8 mm, H 6.5 mm-6.8 mm, L or R 0.0 mm; for LCV (lateral cerebroventricle), p 0.8 mm, H 4.0 mm-4.5 mm, L or R 1.2 mm-1.3 mm. Electrical stimulating pulse was produced by electrotonic stimulator (type SEN-3201, NIHON, KONDON), passing through isolator, then got constant current output.
Stimulating electrode: unipolar co-axil electrode was used, with a diameter of 0.4 mm, length of naked tip 0.1 mm. Stimulating parameters: monophasic spuare wave, 0.3 ms duration, 100 Hz, 0.1 mA-0.3 mA for 2 min. Time between two stimulations: not less than 15 min. For electrolytic lesions, direct positive current 1 mA-1.5mA for 60 s-90 s. Experiments were carried out for 8 min-10 min after electrolytic lesion or LCV injection.
At the end of the experiment, the brain was removed and the position of stimulating and/or lesion sites limits were verified histologically.
Atropine sulfate (No.10 Shanghai Pharmaceutical Factory), phentolamine (CIBA), propranolol (Second Shanghai Pharmaceutical Factory), TRH (Chinese Academy of Med Sci), naloxone (Shanghai Med University).
All data were given as -x±sx and analysed statistically by paired Students’t test, P<0.05 was considered to be significant.
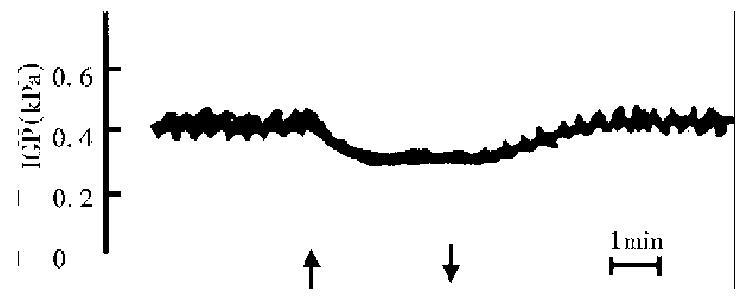
The effects of ARC stimulation on IGP. Before stimulation, IGP was quite stable, with 3 cpm gastric phasic contraction. During stimulation, IGP decreased significantly (42.2% ± 5.4%, n = 15, P < 0.01), the latent period was 6 s-8 s and restored 4.8 min ± 0.7 min after stop of stimulation. The phasic contractions also disappeared during stimulation (Figure 1).
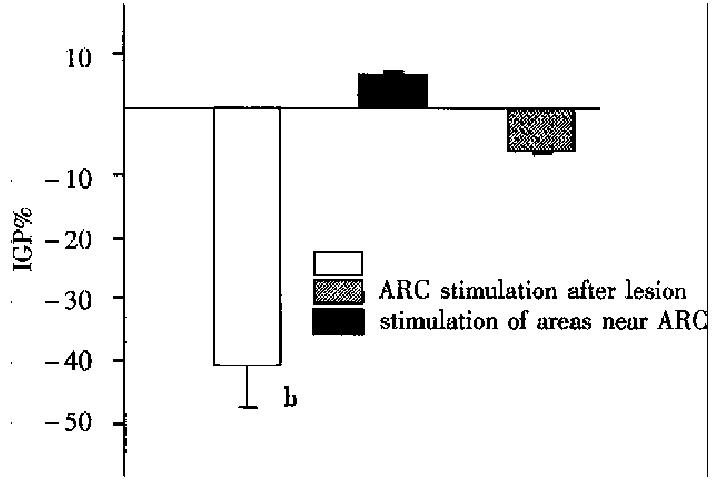
In case the stimulating electrode was not located exactly in the ARC, but near the ARC (n = 7), or stimulation took place after electrolytic action (n = 5), the decreasing effect of stimulation were all non-significant (Figure 2).
The time course of the variation of blood presure during stimulation was not correlated with that of IGP.
The above results indicated that the depression effect of IGP during stimulation of ARC unique special, neither due to the diffusion of stimulating current nor to the changes of cardiovascular activities.
Centrally, lesion of LC or DR led to IGP decrease by 15.7% ± 3.6% and 19.6% ± 2.5% ( P < 0.01, P < 0.05) compared with control group. After intra-cerebroventricular injection of naloxone, the decreasing effect was not changed.
These results showed that both LC and DR, and β-endorphinergic neurons rich in ARC, were involved in the central mechanism (Table 1).
Periphrally, after vagotomy, the decreasing rate of IGP was 24.3% ± 3.2% ( P < 0.05) compared with the corresponding group, but the decreasing effect was not abolished by i. m. atropine. Extirpation of celiac neural plexus or phentolamine i. m could obviously reduce the decreasing effect, while propranolol i. m) did not. After vagotomy plus sympathectomy, the decreasing effect still existed. Such results indicated that the peripheral pathway of decreasing effect of ARC on IGP were through both sympathetic and vagus nerve. The fibers in vagus mediating the reduction of IGP may be non-cholinergic. Humoral factors may also be involved in the peripheral mechanisms (Table 2).
| Treatment | n | IGP(%) |
| Stimulation of ARC | 15 | -42.2 ± 5.4 |
| Normal saline | 10 | -39.1 ± 5.0 |
| Sham operation | 6 | -40.2 ± 8.0 |
| Cervical vagotomy | 13 | -25.7 ± 3.4a |
| Subdiaphragmatic vagotomy | 8 | -24.3 ± 2.2a |
| Atropine | 9 | -30.6 ± 5.0 |
| Extirpation of celiac nerve plexus | 11 | -19.1 ± 3.8a |
| Phentolamine | 7 | -22.8 ± 5.2a |
| Propranolol | 8 | -44.4 ± 6.5 |
| Cervical vagotomy + extirpation | 8 | -12.9 ± 3.9b |
| of celiac nerve plexus | ||
| Subdiaphragmatic | 5 | -15.3 ± 3.8a |
| vagotomy+phentolamine |
At receptor level, α-receptor antagonist phentolamine did not significantly increase the IGP of intact rats, but obviously decreased the IGP of rats with vagotomy. β-receptor antagonist propranolol increased the IGP of both intact and vagotomized rats (Table 3, Table 4).
| Drug | n | IGP (kPa) | ||
| BT | AT | % | ||
| Normal saline | 9 | 0.735 + 0.088 | 0.735 + 0.078 | + 0.6 + 1.6 |
| Phentolamine | 8 | 0.725 + 0.078 | 0.735 + 0.078 | + 2.3 + 2.7 |
| Propranolol | 8 | 0.892 + 0.069 | 0.960 + 0.059 | + 7.9 + 2.3a |
| Treatment | n | IGP (kPa) | ||
| BT | AT | % | ||
| Sham operation + | ||||
| phentolamine | 7 | 0.617 ± 0.098 | 0.588 ± 0.088 | -1.1 ± 2.2 |
| Vagotomy + | ||||
| phentolamine | 7 | 0.8333 ± 0.157 | 0.707 ± 0.137 | -15.6 ± 5.1a |
| Sham operation + | ||||
| propranolol | 4 | 0.568 ± 0.069 | 0.637 ± 0.069 | +17.6 ± 2.5 |
| Vagotomy + | ||||
| propranolol | 5 | 0.784 ± 0.176 | 0.970 ± 0.216 | +28.3 ± 7.9 |
Such results showed that tonic action of adrenergic nerve in stomach was mainly inhibitory. β-receptor may be present on stomach muscle and mediated inhibition. β-receptor, which came into play through vagus, mediated inhibition while those presenting on the stomach wall mediated sympathetic excitation.
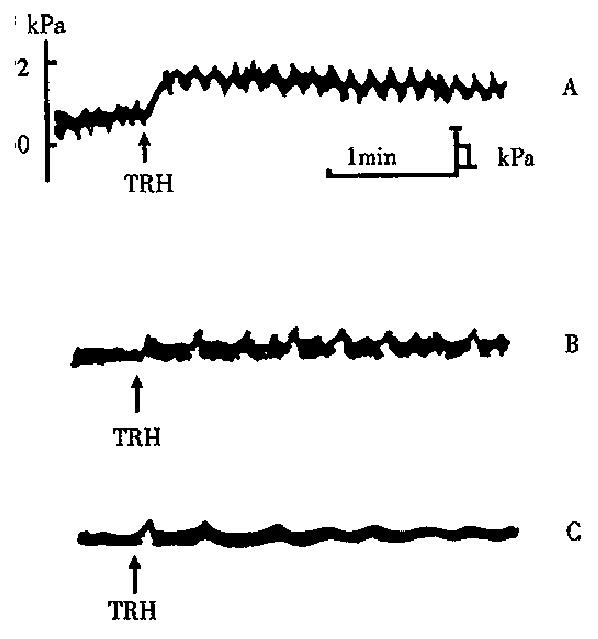
Microinjection of thyrotropin releasing hormone (TRH) 10 μg/1 μL per rat into ARC within 1 min could increase IGP (0.53 kPa ± 0.08 kPa vs 1.5 kPa ± 0.06 kPa) by 183.02% ( n = 10, P < 0.001), latent period 8 s-10 s, lasting 40 min-50 min. The amplitude and frequency of phasic contractions also increased (3 cpm-4 cpm vs 6 cpm) (Figure 3).
ARC is a very complex nucleus, both of its morphology and physiology.
Morphologically, ARC communicates extensively with its neighboring nuclei of thalamus; hypothalamus; midbrain; brainstem; pituitary gland; and limbic system; and also with extensive intrinsic organization the arcuate cells are small and fusiform, or in more lateral position larger and polygonal. The ARC contains many transmitters and peptides and shows phenomena of colocalization of transmitters.
Physiologically the ARC involved in integrating emotional, endocrine, sensory and pain processing and vegetative homeostatic autonomic functions (chronwall, DEPTLDES, 6: Suppl 2, 1-11, 1985). In this study, we showed that the ARC could modulate gastric motility biphasically, both inhibitory and excitatory, The underlying mechanisms responsible for such differences e.g whether due to the different nature of stimulus, and/or the excitation of different ARC cells, and/or liberation of certain transmitters. etc, await further investigations.
It was reported that the effects of adrenergic drugs on the gastro intestinal smooth muscle depond on the nature and location of the receptors. β-receptor, located in the smooth muscle, after i.m. β-receptor antagonist-propranolol, the IGP showed obviously increased, indicated that the β-receptor mediated inhibition. α-receptor, is located both in the nerve plexus of gastric wall as well as in the smooth muscles, the former one by means of pre-synaptic modulation inhibite the release of ACh from the vagus nerve endings, hance inhibite the gastric motility, The α-receptor depend upon the presence of intact vagus. This demonstrated that α-receptor located in nerve plexus can mediate inhibition. In intact rat, after i.m. α-receptor antagonist phentolamine, the IGP only showed some increase, but without statistically significant, this result can be explained as follows: in intact animal with intact vagus nerve, all α-receptors, both in the nerve plexus and smooth musdle were blocked, The two opposite actions, excitation and inhibition cancelled out each other (Table 3); while in the vagotomized rats, those α-receptor located in nerve plexus loss their acting target, the inhibitory action could not expressed, so, under the action of phentolamine, the blocking action of those α-receptors, located in the smooth muscles result in the decrease of IGP (Table 4). This indicated that in intact animal, those α-receptors located in the smooth muscles, mediate excitation. In short, there are three types of receptors: α-excitatory, -inhibitory, and β-inhibitory. These conclusions came from in vitro experiments and used receptor ligands, e.g. adrenaline and noradrenaline, such drugs may alter the physiological body function, thus the conclusion obtained may not be fitted to the normal intact organism.
In view of this, in the present study, we used in vivo experiment and specific antagonists in an attemp to analyse the adrenergic receptors on the gastric wall in intact organism under physiological condition.
| 1. | Ma R, Xu GY. Anatomy and physiology of the hypothalamic arcuate nucleus. Acta Univer Med Anhui (in Chinese)1992,27(2):162-164. . |
| 2. | Ma R, Zhang JX, Xu GY, Qian XJ. Effect of electrical stimulation of hypothalamic arcuate nucleus on intragastric presure in rats. IBID. 1989;24:6-8. |
| 3. | Ma R, Xu GY. Regulation of arcuate nucleus on intragastric pressure in rats. Chin J Appl Physiol (. in Chinese). 1991;7:272-273. |
| 4. | Ma R, Xu GY. [Involvement of locus coeruleus and dorsal raphe nucleus in the reduction of intragastric pressure induced by ACR stimulation]. Shengli Xuebao. 1991;43:489-493. [PubMed] |
| 5. | Ma R, Ling WH, Zhang JX, Xu GY. No effect upon cerebroventricular injection of naloxone on reduction of intragastric pressure induced by stimulation of arcuate nucleus in rats.Acta Univer Med Anhui (. in Chinese). 1990;25:92-93. |
| 6. | Ma R, Xu GY. [Effects of stimulation of arcuate nucleus on intragastric pressure and peripheral pathway analysis in rats]. Shengli Xuebao. 1991;43:376-382. [PubMed] |
| 7. | Ma R, Xu GY. [An investigation on adrenergic receptors in rat stomach]. Shengli Xuebao. 1990;42:397-400. [PubMed] |
| 8. | Cao Q, Xu GY, Qian XJ, Zhang JX. Gastric motive regulation to microinjection of TRH into ARC in rats. A cta Univer Med Anhui (. in Chinese with English abstract). 1991;26:161-164. |