Published online Oct 15, 1998. doi: 10.3748/wjg.v4.i5.404
Revised: August 19, 1998
Accepted: September 12, 1998
Published online: October 15, 1998
AIM: To evaluate the immunity of chemically modified tumor cell vaccine.
METHODS: Tumor cell vaccines (TCV) were prepared by incubating the live Ehrlich ascites tumor cells with concanavalin A-mitomycin C (ConA-MMC), mitomycin C (MMC), concanavalin A-glutaraldehyde (ConA-Glu), glutaraldehyde ( Glu ), or paraformaldehyde ( Para ), respectively. The whole cell or soluble forms of the vaccines were administered intraperitoneally into Kunming mice once a week for three times prior to the intraperitoneal inoculation of a lethal dose of live tumor cells. A second challenge with live tumor cells was given four weeks later. Survival and antibody production of the mice were analyzed.
RESULTS: After the first challenge, the mice, received whole TCV of ConA-MMC, MMC (P < 0.01) and Glu (P < 0.05) promoted survival incidence than the controls. All the treated mice had the survival time prolonged. ConA-MMC vaccine treated mice had longer survival days than that of ConA-Glu ones (P < 0.05). For the soluble TCV immunized mice, those treated with vaccines of Para (P < 0.01), ConA-Para and ConA-Glu (P < 0.05) had longer survival periods compared with that of the controls. Following the second challenge, survival incidence of the mice received vaccines of ConA-MMC, MMC, ConA-Glu or Glu was significantly increased (P < 0.01). Moreover, all the treated mice had the survival time prolonged, and ConA-MMC vaccine treated mice had longer survival days than that of Para treated ones (P < 0.05). Antibodies against Ehrlich ascites tumor cells were found to be positive in sera of the mice treated with whole TCV of ConA-MMC.
CONCLUSION: Ehrlich ascites tumor cells are immunogenic when treated with ConA-MMC, MMC, ConA-Glu, Glu or Para, which might act as safe and effective tumor vaccines with safety and effectiveness.
- Citation: Ma Z, Zhou SJ, Wu KC, Pan BR, Qiao TD, Chen BJ, Fan DM. Immuno-protective effect of tumor cell vaccine on Kunming mice bearing Ehrlich ascites tumor. World J Gastroenterol 1998; 4(5): 404-408
- URL: https://www.wjgnet.com/1007-9327/full/v4/i5/404.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i5.404
Biotherapy represents an alternative option after surgery, radiotherapy and chemotherapy for cancer. Tumor vaccine and the induction of active specific immunity are of great interest in this field. However few tumor antigen isolated from tumor cells has been identified as the targets for immune system, except for some from the melanoma. Early animal experiments indicated that tumor cell antigens should be presented as a certain form to induce the protective immune response. Modification of tumor cells by different methods as tumor vaccines applies to all kinds of tumor types under the condition of unidentified tumor antigen. This method is simple, cost-effective and useful in clinic practice[1]. The aim of this study is to investigate the possibility and feasibility of this kind of modified tumor vaccines.
Ehrlich tumor cells were obtained from the Animal Center of the Fourth Military Medical University, which passed on by inoculating up to 2 × 106 cells in 0.1 mL suspension intraperitoneally into naive mice. Tumor cells were collected from ascites and incubated with mitomycin C ( MMC ) at a concentration of 20 pg/cell at 37 °C for one hour, or with glutaraldehyde (Glu) at concentrations of 0.12 mL/L-0.25 mL/L on ice for 30 min, or with 40 mg/L Para (the final concentration was 20 mg/L) on ice for one hour, then followed by washing cells for 3 times by centrifugation at 800 ×g with normal saline (NS). The modified tumor cell sample is termed as MMC-Tu, Glu-Tu and Para-Tu vaccines, respectively. MMC-Tu/Glu-Tu/Para-Tu cell vaccines were mixed with 1g/L Concanavalin A (ConA, the final concentration was 200 mg/L) on ice for one hour, washed for 3 times, and then referred to ConA-MMC Tu, ConA-Glu Tu and ConA-Para Tu vaccines, respectively. For preparation of soluble TCV, the whole TCV cells were suspended in antigen extract buffer (0.01 mol/L Tris-HCl containing 0.5 mol/L NaCl, 1 mmol/L EDTA, 2.5 mL/L NP-40). After being ultrasonicated with 20 Hz at 4 , for 10 min × 10 times, cells were centrifuged at 12000 ×g for 60 min at 4 °C. The supernatant was passed througha 0.22 μm filter. The soluble antigens amounting to 107 cells/mouse were emulsified with Freud’s adjuvant of the same volume.
Female Kunming mice aged 8 weeks were provided by the Animal Center of the Fourth Military Medical University, divided into MMC-Tu, ConA-MMC-Tu, Glu-Tu, ConA-Glu-Tu, Para-Tu and ConA-Para groups (for soluble TCV only) randomly. Each mouse was inoculated intraperitoneally with 107 of whole TCV cells in 0.2mL of suspension at day 1, 12 and 19. The controls were given 0.2 mL of NS. Two weeks after the last inoculation, each mouse was challenged intraperitoneally by injection of 107 (lethal dose) of live tumor cells. The survivals of free tumor one month after the 1st challenge were repeatedly inoculated with 1.44 × 107 of live tumor cells. The mice were observed daily within 2 months. The soluble TCV were inoculated intraperitoneally into mice at day 1, 21 and 28. After 2 weeks of the last vaccination, all the mice were challenged with 107 of live tumor cells. The mice were observed daily within one and a half months. Therapeutic response was evaluated by promotion of incidence of survivals and by prolongation of life span of each mouse. The survival days were expressed as -x±s.
The results were analyzed for significance using the ANONA in multiple experiments for prolongation of life span and Fisher exact test with SPLM software for survival incidence.
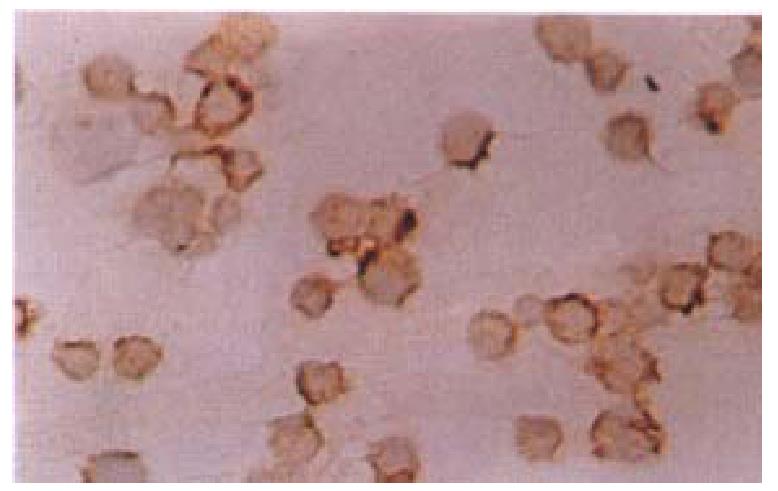
The tumor cell smears were prepared and fixed in acetone, followed by treating in methanol containing 0.3 mL/L H2O2 at room temperature for 30 min. After washing with PBS for 3 times, the cells were blocked with 100 mL/L FCS at 37 °C for 30 min, then incubated with the serum collected from the mice immunized with the whole ConA-MMC-Tu TCV as primary antibodies overnight at 4 °C, washed 3 times with PBS and covered with a biotin-conjugated secondary antibody for 30min at 37 °C. For the staining, an avidin-biotin-complex HRP was used (vector). For the controls, normal mouse sera were used instead of the primary antibodies.
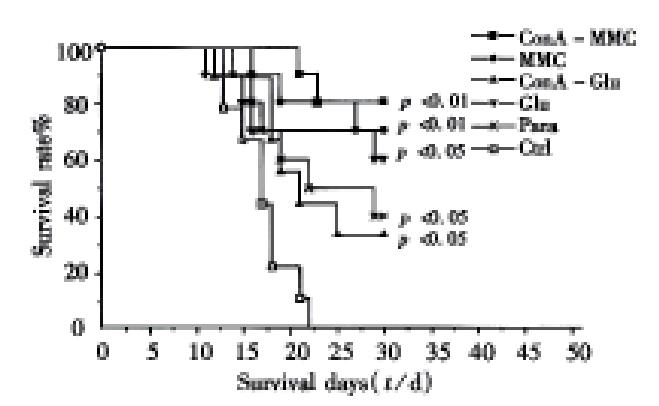
There was no evidence of tumorigenesis such as ascites or palpable tumor node subcutaneously when the modified TCV was injected into the peritoneal cavity of mice. By the end of day 30, 8/10 ConA-MMC mice, 7/10 MMC mice, 6/10 Glumice, 3/9 ConA Glu mice and 4/10 Para mice survived from the first tumor challenge, whereas none of the controls did. The survival of the mice in ConA-MMC, MMC groups (P < 0.01) and Glu group (P < 0.05) has been prolonged significantly as compared with the controls (Table 1). At the same time, there was significant difference in the survival days of the mice in ConA-MMC group (28 d ± 3 d), MMC group (27 d ±5 d) (both P < 0.01), ConA-Glu group (23 d ± 7 d), Glu group (25 d ± 8 d) and Para group (24 d ± 7 d) (all P < 0.05) as compared with 17 d ± 3 d of the controls ( F = 4.55, P = 0.00164, Figure 1). Among all the treated mice, the ConA-MMC group appeared to have longer survival time than ConA-Glu group (t = 2.49, P = 0.02). Antibodies against the cellular membrane of Ehrlich ascites tumor cells were positive in the sera of the mice immunized with the whole TCV of ConA-MMC for three times about 35 days (Figure 2).
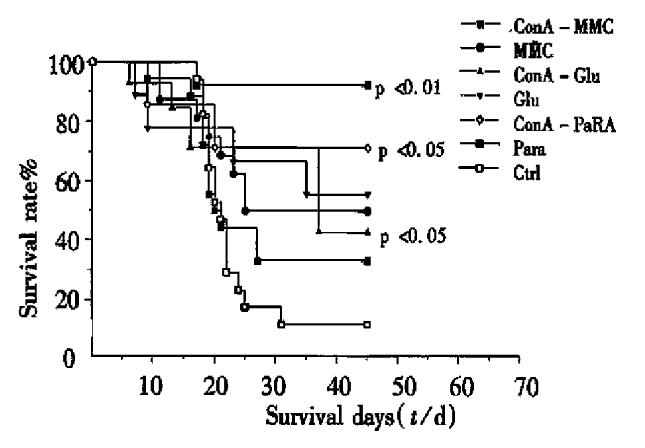
The survival days and rates of the mice in groups of MMC, Glu (P < 0.05), ConA-Para and Para (P < 0.01) were obviously different from that of the control ( Table 2, F = 3.23, P = 0.00653, Figure 3). Among them, ConA-Glu, ConA-Para (both P < 0.05) and Para (P < 0.01) treatment conferred the immunity to prolong the life span of the immunized mice, particularly the Para TCV treated mice (longer than those treated with ConA-MMC, MMC and ConA-Glu, all P < 0.05).
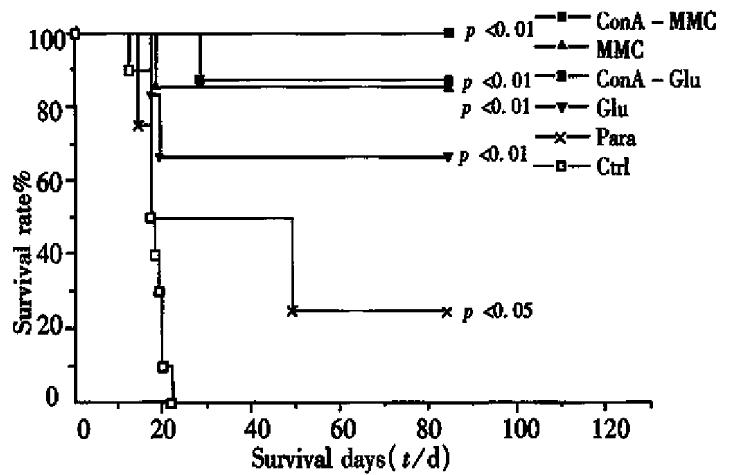
The survivors of the whole TCV immunized individuals after the first challenge free of tumor signs (i.e., increase of ascites fluid and/or delayed palpable S.C. tumor growth) received the second challenge with live tumor cells. ConA-MMC mice 7/8, MMC mice 6/7, Glu mice 4/6, ConA-Glumice 3/3 and Para mice 1/4 survived as long as 12 weeks, whereas all the controls died within 1 month (Table 3). The survival rate of all the treated groups, except Para, was significantly different from the control (P < 0.01). All the vaccines prolonged the survival periods except the control (Para group P < 0.05, other groups P < 0.01, Figure 4). The survival periods of the mice in groups of ConA-MMC, MMC, ConA-Glu, Glu and Para were 77 d ± 20 d, 75 d ± 25 d, 84 d ± 0 d, 62 d ± 34 d and 41 d ± 33 d, which were significantly different from 18 d ± 3 d of the controls ( F = 9.83, P = 9.2 ± 10-6). ConA-MMC group showed a stronger response than Para group (t = 2.41, P = 0.04).
The results have provided important evidence that chemicals have exerted some in fluences on tumor cells and manifested in the following aspects. All the treatment could make tumor cells lose their tumorigenesis. No evidence of ascites was observed in mice inoculated with the treated tumor cells. Combination analysis of the results after 2 times of challenge revealed that ConA-MMC, MMC or Glu treatment has a consistent tendency of prolonging the life span of immunized mice and increasing the incidence of survival. And administration of such vaccines could protect for as long as 12 weeks, in which memory cells may be involved. Although mice with ConA-Glu treatment for twice both had longer span than the controls, the survival rate was improved only after the second challenge (P = 0.00035). Para treatment could prolong the survival period but not increase the rate after two times of tumor challenge. The whole TCV, therefore, was able to promote the immunized mice with a higher threshold of resistance to lethal dose of live tumor cells.
Previous studies indicated that both ConA-MMC and ConA-Glu treated L1210 leukemia cells could resist the challenge of tumor cells, and ConA-MMC-Tu vaccine was more potent immunoprophylactics in inducing immune resistance of mice than ConA-Glu vaccine[2]. Our study showed that both types of vaccines with Ehrlich ascites tumor could prolong the life span of survivors, but as for short-term immune response, ConA-MMC had longer survival days than ConA-Glu (P < 0.05), and as for long-term one, there was no difference between the two, possibly because of the different tumor models.
We found that the immunized mice contained antibody against cellular membrane of the tumor cells, but whether it is a specific antibody against a tumor antigen, H-2 allele or a common cellular component remains to be elucidated. Intravenous injection of paraformaldehyde-fixed autologous cells infected in vitro with recombinant vaccinia virus expressing HIV-1 protein gave high levels of neutralization antibody, cell-mediated cytotoxicity and DTH response[3]. Recently Shrayer et al[4,5] reported that the immunized mice could acquire cytotoxic antibodies when injected with paraformaldehyde or glutaraldehyde treated tumor vaccines. The antibodies not only inhibited the proliferation of the tumor cells, but also eradicated the tumor cells and exerted the protective immune response by the mechanisms of ADCC and CDC. Similar results were obtained with Lewis lung carcinoma[6]. Further studies to determine whether the antibodies are cytotoxic are being carried out.
The soluble TCV showed different results from those of the whole TCV. Although ConA-Para treated whole cells had a marginal vaccine effect, the soluble fragments showed the most obvious one. On the contrary, the soluble ConA-MMC TCV did not show the expected vaccines effect as the whole one did. Whether the procedure of sonication released some “new” immunogenic determinants that existed originally in the cytoplasm or nucleus, or whether it destroyed some antigens remains to be investigated in detail.
Conventional wisdom holds that the MMC could bind the DNA of tumor cell and totally block DNA synthesis especially targeted G1 and S phases, which resulted in losing the tumorigenesis and exposing “new” TAA determinants. Formalin treatment not only stopped cell division, but also kept the integrity and preserved the antigenicity of the tumor cells. Though less liable in solubilization procedures, formalin-stabilized surface antigens could be solubilized through sonication. Other studies assumed that the increased efficacy of formalin was due to exposed aldehyde groups, resistance of aldehyde cross-links to hydrolysis at low pH, and the nature of cross-linking of antigen monomers[4]. The treatment of further modification by ConA could enhance the interaction of tumor cells and immune cells[7].
Kunming mice were developed in China and obtained the acknowledgement of the world. But till now their genetic background was not known. There might exist slight all ogeneic difference between individuals. Actually such treated tumor cells as vaccines is a kind of incomplet allogeneic tumor cell vaccines when injected into the individuals who had haplotype difference between the recipients and donors. Allogeneic tumor cell vaccines, either sharing at least one MHC class I restricting element (incompletely) or not matching for any MHC molecule (completely), can induce syngeneic protective T cell-mediated antitumor response, instead of deducing the responses[8]. The phenomenon is explained by the theory of cross priming.
In our study, the immune response to the vaccines may represent a combination of the immunity to the tumor antigens as well as the allogeneic antigens. In fact, the present study showed that there existed the discrepancy of individual reaction to the same tumor vaccines. On the basis of the common Ehrlich ascites tumor cells vaccine, the greater the difference is, the stronger the immunity is[9].
Ehrlich ascites tumor cell belongs to undifferentiated type. It was established in 1932 by injection of saline containing spontaneous breast adenocarcinoma into peritoneum. The characteristics of this tumor cell line are low immunity, being transplantable and spontaneous origin, similar to that of the non-virus originated human tumor[10].
In conclusion, tumor cells treated with chemicals or antitumor drugs can be used as tumor vaccines as indicated by production of many cured animals and prolongation of life span of immunized mice. However the cellular or humoral mechanism awaits further studies.
Project supported by the Outstanding Youth Fund from National Natural Sciences Foundation of China, No.39525020.
| 1. | Mallmann P. Tumor vaccination. Hybridoma. 1995;14:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Kataoka T, Ogihara K, Sakurai Y. Immunoprophylactic and immunotherapeutic response by concanavalin A-bound tumor vaccine enhanced by chemotherapeutic agents eliminating possible suppressors. Cancer Res. 1980;40:3839-3845. [PubMed] |
| 3. | Kataoka T, Oh-hashi F, Tsukagoshi S, Sakurai Y. Induction of resistance to L1210 leukemia in BALB/c X DBA/2Cr F1 mice, with L1210 cells treated with glutaraldehyde and concanavalin A. Cancer Res. 1977;37:964-968. [PubMed] |
| 4. | Shrayer D, Kouttab N, Maizel A, Wanebo H, Hearing VJ, Gersten DM. Generation of cytotoxic antibodies to the B16 murine melanoma using a formalinized vaccine. Int J Cancer. 1993;53:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Shrayer D, Bogaars H, Hearing VJ, Maizel A, Wanebo H. Further characterization of a clinically relevant model of melanoma metastasis and an effective vaccine. Cancer Immunol Immunother. 1995;40:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Veltri RW, McKolanis JR, Rockoff SD, McIntire KR. Protein-A antibody-binding TPAAB) technique for detection of tumor-associated membrane antigen (TAMA) of Lewis lung carcinoma. Int J Cancer. 1980;25:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Toes RE, Blom RJ, van der Voort E, Offringa R, Melief CJ, Kast WM. Protective antitumor immunity induced by immunization with completely allogeneic tumor cells. Cancer Res. 1996;56:3782-3787. [PubMed] |
| 8. | Bevan MJ. Antigen presentation to cytotoxic T lymphocytes in vivo. J Exp Med. 1995;182:639-641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Zagury D, Bernard J, Cheynier R, Desportes I, Leonard R, Fouchard M, Reveil B, Ittele D, Lurhuma Z, Mbayo K. A group specific anamnestic immune reaction against HIV-1 induced by a candidate vaccine against AIDS. Nature. 1988;332:728-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 152] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Shi XY. Animal medical experimental methods (in Chinese). Beijing: People's Medical Publishing House. 1980;227-232. |