Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.343
Revised: March 18, 1998
Accepted: April 16, 1998
Published online: August 15, 1998
AIM: To compare and analyze the contrast enhancement appearance of small hemangioma (SHHE) and small hepatocellular carcinoma (SHCC) with helical multi-phase CT scanning so as to determine their roles and pitfalls in the differential diagnosis of SHHE and SHCC.
METHODS: The pre and postcontrast CT scanning of the liver in 73 cases (38 SHHE, 35 SHCC) were carried out. The first phase scan of the entire liver began at 30s after the injection of contrast medium, the second and third phases began at 70s, and 4 min respectively. The contrast enhancement patterns and characteristics of all lesions were observed and compared.
RESULTS: In SHHE, 64.29% (27/42) had typical manifestations in two-phase dynamic scanning, such as peripheral dramatic high-density enhancement of the lesions with progressive opacification from the periphery toward the center, 30.95% (13/42) were hyperdense in both phases and 4.76% (2/42) were hypodense in both phases. In the third phase scanning, 96.67% (28/30) of SHHE were hyperdense and isodense. In SHCC 59.52% (25/42) presented typical appearances, such as hyperdense in the first phase and hypodense in the second phase, 23.81% (10/42) were hyperdense in the first phase and isodense in the second phase with 4.76% (2/42) of hypodense in both phases. In the third phase scanning, 85.71% (24/28) of SHCC were hypodense.
CONCLUSION: According to the contrast enhancement patterns of SHHE and SHCC in the two-phase or multi-phase scanning by helical CT, diagnosis can be established in the majority of lesions, while some atypical cases needed MRI for further investigation.
- Citation: Yan FH, Zeng MS, Zhou KR. Role and pitfalls of hepatic helical multi-phase CT scanning in differential diagnosis of small hemangioma and small hepatocellular carcinoma. World J Gastroenterol 1998; 4(4): 343-347
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/343.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.343
Hemangioma is the most common benign tumor of the liver. It is found incidentally during the abdominal imaging examination, which is important in the differential diagnosis from hepatocellular carcinoma (HCC)[1] . In most cases, the differential diagnosis is easy, but in some cases, it is difficult. With the application of helical CT, the scan speed is greatly improved, the entire liver can be scanned during a single breath-hold and it can be done during dual-phases or multi-phases of hepatic enhancement for optimal visualization of small hemangioma (SHHE and small hepatocellular carcinoma (SHCC)[2]. To improve the capability of differential diagnosis between SHHE and SHCC, it is necessary to renew their enhancement patterns on helical CT scanning. We analyzed the appearance of 38 SHHE (42 lesions) and 35 SHCC (42 lesions) on dual-phase and multiphase helical CT scanning, and evaluate their roles and pitfalls in the differential diagnosis between the two.
Seventy-three patients were reviewed, 52 men, 21 women, aged 32-76 years, averaging 53 years. Thirty-five cases of SHCC were confirmed by surgical pathology or biopsy and 10 of 38 cases of SHHE were proved by surgery, and the others confirmed with the combination of other examinations (US, MRI) and the follow-up CT scan at least for one year. All lesions were ≤ 3 cm in diameter. The CT imaging was performed with a GE-Hispeed Advantage Scanner, scanning time 1s, 120 kV-140 kV, 230 mA-280 mA, an image matrix of 512 × 512, section thickness and interscan gap of 5 mm or 10 mm. The postcontrast scanning was started after the plain scan. The contrast medium (CM, Ultravest) was injected at a rate of 3 or 4 mL/s with a total amount of 80 mL-120 mL (1.5 mL/kg body weight). The first (arterial) phase scan of the whole liver began at 30 s after initial injection of CM into the antecubital vein, and the second (portal venous) phase scan started at 70 s. In addition, 28 SHCC patients and 30 SHHE patients underwent the third (delayed) phase scan with 4 min.
All cases received US examination (HITANICA-EUB-40B, Japan), and 25 of the patients underwent MR imaging (GE-1.5T Sigma Superconducting Magnet). The SE technique was used and T1-weighted and T2-weighted (2000/30-50) imaging of the entire liver was carried out. The dynamic contrast enhancement MR imaging was performed with the fast multiplanner spoiled gradient recalled sequence.
Fourty-two lesions were detected in 38 SHHE patients with a size of 1 cm-3 cm, averaging 2.2 cm,and 42 lesions were detected in 35 SHCC patients, with a size of 0.8 cm-3.0 cm, averaging 1.8 cm.
Twenty-eight lesions of SHHE were detected, 25 of them had low attenuation with the edge clear or unclear, 3 of them presented high attenuation because of the existence of fatty liver. Twenty-six SHCC were detected on the plain scan, 24 of them had low attenuation and 2 had high attenuation with fatty liver background.
On the first phase, 30.95% (13/42) of the SHHE revealed homogeneous high attenuation, 50% (21/42) of them with unhomogeneous high attenuation, and 19.05% (8/42) homogeneous low attenuation. On the second phase, 61.90% (26/42) presented with homogeneous high attenuation, 28.57% (12/42) with unhomogeneous high attenuation, 2.38% (2/42) isoattenuation and low attenuation, respectively.
On the first phase scan, SHCC presented the following enhancement findings: homogeneous high attenuation in 71.43% (30/42), unhomogeneous high attenuation in 16.67% (7/42), isoattenuation in 7.14% (3/42) and low attenuation for 4.76% (2/42). On the second phase, 71.43% (30/42) of them became low attenuation (homogeneous or unhomogeneous), 23.81% (10/42) isoattenuation, 4.76% (2/42) had high attenuation associated with fatty liver.
On the delayed phase scan, the appearance of SHHE varied, but the majority (96.67%) were hyperdense (homogeneous or unhomogeneous) and isodense, and the other 2 of the 30 were homogeneously hypodense, one of them was hypodense, the other associated with fatty liver was hyperdense on both plain scan and the first phase scan, and became hypodense compared with the liver parenchyma enhanced markedly on the second phase. Those two cases were misdiagnosed as hepatocellular carcinoma, but on the dynamic MR contrast imaging, they began to be opacified fully at the 10 min. For SHCC, 85.71% (24/28) were hypodense on the delayed phase scan, 4 were isodense (with fatty liver), 2 hyperdense on the first and the second phase, and the other 2 were hyperdense on the first phase and isodense on the second phase, resulting in misdiagnosis of SHHE preoperatively.
The diagnostic standards of HHE suggested by Freeny[3] were: (1) early contrast enhancement in the peripheral areas; (2) progressive opacification from the periphery to the center of the lesion; (3) full opacification in the delayed scan, but only 55% HHE were typical due to the different characteristics of each lesion and pitfalls of the scan protocol. The single-level dynamic CT can remedy the shortcomings of conventional contrast CT, but the scan phase can not be controlled accurately to pass through the center of small lesions. The helical CT is able to overcome the disadvantages of the conventional dynamic CT. The scanning of entire liver can be finished during a single breath-hold without respiration motion. On the other hand, the major advantage of helical CT is that the entire liver can be scanned at the different periods of hepatic enhancement, facilitating lesion’s characterization in general[4]. The arterial phase scan usually started at 25 s-35 s after initial injection of contrast medium, and the portal phase at 65 s-70 s, sometimes an additional delayed scan at 3 min-5 min was needed in patients suspected of having HHE in order to distinguish from HCC.
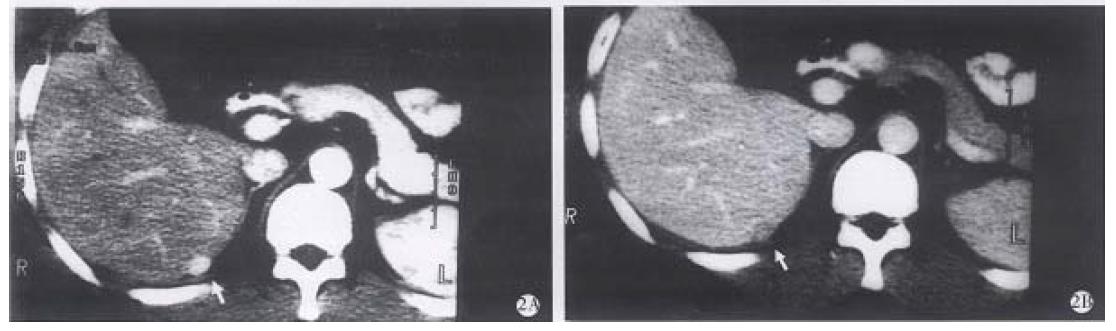
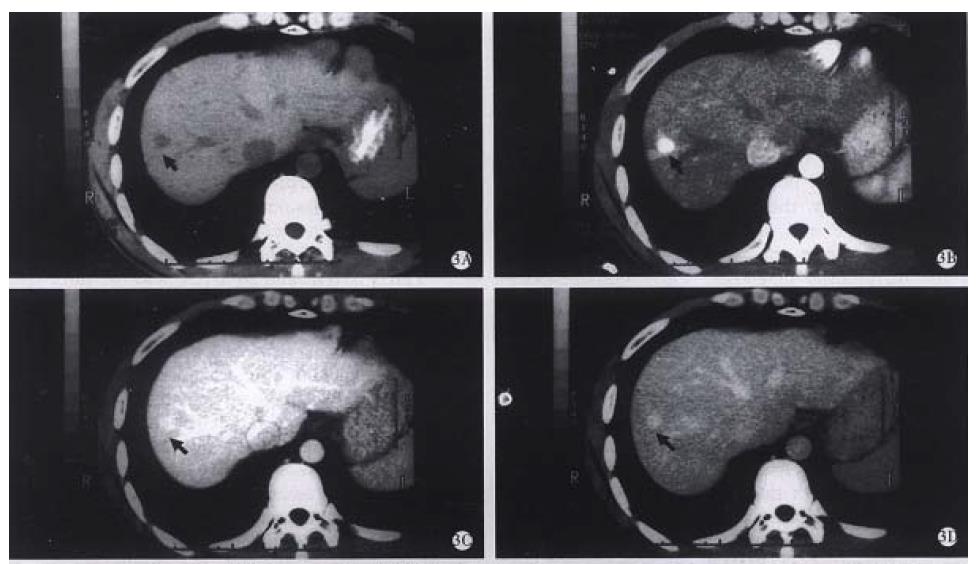
Our results showed that, 27 of 42 SHHE (64.28%) had obvious globular enhancement peripherally (Figure 1A, B), and it was not seen in the other 15 lesions. Thirteen of 15 lesions were homogeneous hyperdense on the early phase, which was difficult to differ from hyperwascular SHCC, but in the second phase, the lesions remained hyperdense, which was helpful in making definite diagnosis of SHHE. The time of opacification needed in SHHE was usually 1 min-4 min or more, but the smaller the lesion, the faster the spreading of contrast medium, becoming homogeneously hyperdense rapidly, with a longer peak period of about 1 min-2 min[1,5] , therefore, remaining hyperdense on the second phase scan. Thirty-seven of 42 of SHCC (88.09%) cases were homogeneous or unhomogeneous hyperdense on the first phase, but 71. 43% of them became hypodense on the second phase (Figure 2A, B), and 23.81% (10/42) of them were isodense. The result showed that the peak period of early enhancement of SHCC lasted short and waked out rapidly, so the dual-phase (arterial phase and portal venous phase) scans can reflect sufficiently the specific feature of blood supply of SHHE and SHCC. Findings during the portal venous phase scan was especially important for the differential diagnosis. Early homogeneous hyperdense findings in some SHHE have been reported to be atypical[1-7], but the prolonged hyperdense on the second phase can make a positive diagnosis (Figure 3 A-D). In addition, Hanafusa et al[5] reported a group of SHHE, 7 of them were considered atypical as hyperdense on the first phase and isodense on the second phase. Ten lesions in our group had the same appearance, all were performed with the delayed phase scan, 8 of them being hypodense and diagnosed as SHCC (Figure 4A -D). The other two lesions of isodense were misdiagnosed as SHHE preoperatively, but later were confirmed as SHCC by the surgical pathology. The misdiagnosis might be inferred that a few SHCC received the blood supply from portal vein rendering them more hypervascular and prolonged enhancement into the portal venous phase[8]. On the other hand, in the patients with fatty liver, due to the lower parechymal attenuation, the difference between the lesion and the liver parenchyma was minimized. The delayed phase scan was necessary in these patients, so as to lower the misinterpretation. The demonstration of capsule of the lesions was also important in the differential diagnosis, but rarely seen on CT, and MR imaging may be helpful under this condition.
Thirty SHHE and 28 SHCC had the third phase scanning, the result showed that 96.67% (20/30) of SHHE were hyperdense or isodense, and 85.71% (24/28) of SHCC were hypodense. These reflected further changes of the enhancement patterns of SHHE and SHCC. The early enhancement patterns of SHHE were various, but the majority of lesions were opacified gradually or rapidly and lasting longer while those of SHCC had the enhancement pattern of both ascending and descending rapidly. In some atypical cases, especially in the patients associated with fatty liver, either dual-phase or multi-phase scan showed the limitation in the differential diagnosis, and MR imaging would be the best choice.
Depending on the enhancement patterns of SHHE and SHCC, the following findings were helpful in the diagnosis of SHHE: (1) peripheral globular enhancement on the first phase; (2) homogeneous hyperdensity on the first phase and maintaining to the second phase or/and the third phase; (3) homogeneous hyperdense on the first phase and isodense on the second phase and the third phase (except the cases associated with fatty liver). Forty of 42 SHHE had the above-mentioned findings (95.24%), the other two was difficult to diagnose.
MRI was considered to be the most accurate method in the diagnosis of HHE among the imaging modalities with an accuracy of 95%[9], helical CT could alsoreflect sufficiently the blood supply of SHHE, thus helping with a definite diagnosis. The result of our study showed that the accuracy of diagnosis with helical CT was similar to that of MRI, and significantly superior to that of conventional CT especially in the portal venous phase scan for the differential diagnosis. Two phase scan should be routinely used, the addition of the third phase scan or multiphase scan would be the choice for further confirmation, and if the diagnosis is still unclear, MRI ought to be the preference.
Project supported by the National Natural Science Foundation of China, No.39282229.
| 1. | Zhong KR. CT of the abdomen. 1st Ed. Shanghai: Shanghai Medical Univer-sity Publisher. 1993;47. |
| 2. | Berland LL. Slip-ring and conventional dynamic hepatic CT: contrast material and timing considerations. Radiology. 1995;195:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Freeny PC, Marks WM. Hepatic hemangioma: dynamic bolus CT. AJR Am J Roentgenol. 1986;147:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Heiken JP, Brink JA, Vannier MW. Spiral (helical) CT. Radiology. 1993;189:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 172] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Hanafusa K, Ohashi I, Himeno Y, Suzuki S, Shibuya H. Hepatic hemangioma: findings with two-phase CT. Radiology. 1995;196:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Gaa J, Saini S, Ferrucci JT. Perfusion characteristics of hepatic cavernous hemangioma using intravenous CT angiography (IVCTA). Eur J Radiol. 1991;12:228-233. |
| 7. | Itai Y, Araki T, Ohtomo K, Kokubo T, Yoshida H, Minami M, Yashiro N. Well-defined, dense and continuously spreading enhancement on single level dynamic CT of the liver: a characteristic sign of hepatic cavernous haemangioma. Rofo. 1989;151:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 371] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Whitney WS, Herfkens RJ, Jeffrey RB, McDonnell CH, Li KC, Van Dalsem WJ, Low RN, Francis IR, Dabatin JF, Glazer GM. Dynamic breath-hold multiplanar spoiled gradient-recalled MR imaging with gadolinium enhancement for differentiating hepatic hemangiomas from malignancies at 1.5 T. Radiology. 1993;189:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 107] [Article Influence: 3.3] [Reference Citation Analysis (0)] |