Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.326
Revised: April 24, 1998
Accepted: May 12, 1998
Published online: August 15, 1998
AIM: To study the thermotherapeutic effects of implanted ferromagnetic thermoseeds in high frequency electromagnetic field in hepatic tumors.
METHODS: The ferromagnetic thermoseeds made of nickel-copper alloy, which has a lower Curie temperature, were implanted into hepatic tumors of mice. The high frequency electromagnetic field was then applied in vitro to make the ferromagnetic thermoseeds produce the hyperthermia. Before and after thermotherapy, the tumor size, pathologic alteration and animal survival period were assessed.
RESULTS: The temperature at the central area of the tumor could be heated up to 50 °C. Most of tumors in mice disappeared with a large amount of tumor necrosis. The survival period of mice was prolonged.
CONCLUSION: This thermotherapy is beneficial to directional selection and temperature control for treatment of hepatic tumors.
- Citation: Geng YC, Wang XX, Ma Y, Hu Y, Zhang RL. Orientated thermotherapy of ferromagnetic thermoseed in hepatic tumors. World J Gastroenterol 1998; 4(4): 326-328
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/326.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.326
The problem of selected hyperthermia for tumors has not been well solved so far. In this paper the orientated heating technique of implanted ferromagnetic thermoseed induced by high frequency electromagnetic field was evaluated in mice hepatic tumors.
Thirty-eight female Kunming mice (provided by Xuzhou Medical College) weighing 18 to 22 g were used . Hepatic tumor models were made by subcutaneous inoculation of H22 cells (provided by Shanghai Institute of Materia Medica, Academia Sinica) to the back of the mice. Tumors were allowed to grow for 1 week. When the tumors were measured to be as large as 10 mm in diameter in 1 week, the animals were used for experiment. Part of them were used to observe the temperature change during the heating of tumors and the histopathological changes after the tumors were heated. The others were divided at random into four groups to observe the growth of tumors and the survival period of the animals.
Untreated control group (G1). Tumor-bearing animals received no treatment.
Thermoseed group (G2). The tumors of the mice were implanted with two ferromagnetic thermoseeds which were 1cm in length in parallel.
Electromagnetic group (G3). Tumor-bearing animals were exposed to high frequency electromagnetic field for 60 min.
Heat-treated group (G4). G4=G2+G3, tumor-bearing animals were kept in a position in which the thermoseed axes must be aligned with the direction of the magnetic field.
High frequency electromagnetic field was produced by the induction coil of our “heating apparatus”. Megnetic field strength at the center of the coil was 5 mT and the frequency was 200 kHz. Water was supplied for cooling the coil during the induced heating.
Ferromagnetic thermoseed (provided by Beijing Science and Technology University) 1 mm in diameter was made of nickel-copper alloy. Induced heating of the seed by eddy current would generate when the seed was exposed to electromagnetic field. Then the seed of this alloy had its Curie point at 60 °C. When the seed was heated up to the Curie point in the magnetic field, the seed would lose its ferromagnetic properties, thereby providing an automatic temperature regulative effect.
A laboratory-built thermometer was used to measure the temperature during hyperthermia. The sensor of the thermometer was made of thermocouple with the error of +/- 0.5 °C. And the sensor was shaped in needle, therefore it could be inserted into tissue directly for measuring the temperature.
The diameter of the tumors was measured 2-dimensionally using a sliding caliper. The average size of a tumor was expressed by the product of maximum diameter and minimum diameter. The statistical differences were assessed using Student’s t test.
Thermoseed-induced heating at the centre of tumors occurred rapidly after the magnetic field was applied. To heat the tumor up to 42 °C, the effective therapeutic temperature in tumor hyperthermia, only 5 min was needed . The maximum temperature to 50 °C was recorded in 30 min and was stabilized in subsequently continous heating . The temperature of the boundary between tumor and normal tissues increased less rapidly and maintained at 39 °C (Table 1).
| Heating time (min) | Centre of tumor | Boundary of tumor |
| 5 | 42.93 ± 1.10 | 34.91 ± 0.81 |
| 10 | 46.90 ± 1.14 | 35.41 ± 1.00 |
| 20 | 47.38 ± 1.52 | 35.94 ± 1.03 |
| 30 | 50.02 ± 2.46 | 38.13 ± 1.42 |
| 40 | 49.67 ± 2.68 | 38.92 ± 2.33 |
| 50 | 49.74 ± 3.12 | 39.22 ± 2.94 |
Coagulation necrosis occurred in the tumors after thermal injury. Tumor tissues for the light microscopic examination were prepared in 1 week after the induced heating was performed. The sections showed that there were extensive necrosis and infiltration of inflammatory cells appeared in the peripheral region.
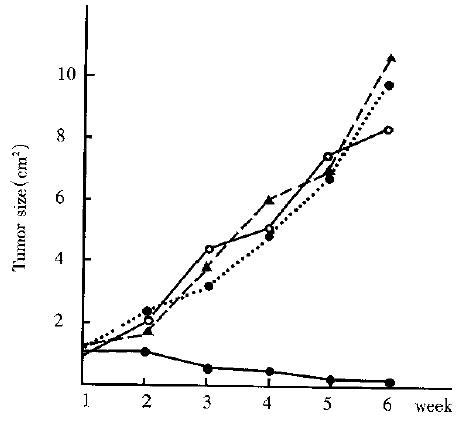
In all groups of animals, there was no significant difference in tumor size before thermotherapy. In the heat-treated group (G4, n = 7) the tumor began to decrease in size in 2 weeks after the induced heating and disappeared in 5 of 7 animals 5 weeks later. And the tumor growth in the remaining two animals was significantly delayed. Meanwhile, the tumor in the other 3 groups (G2, G3, G4) grew progressively (Figure 1). By the time of 5 weeks, the tumor size of G1-G4 was 9.74 cm2± 4. 72 cm2 ( n = 8), 10.71 cm2± 5.16 cm2 ( n = 8), 8. 51 cm2± 3.42 cm2 (n = 8) and 0.15 cm2± 0.17 cm2 (n = 7). The difference was significant in tumor size between heat-treated groups and the control groups (P < 0.01). The survival time in 4 groups is shown in Table 2. All of the animals in the 3 control groups died within 10 weeks. While the survival time of the heat-treated animals was markedly prolonged as compared with the control (P < 0.01). Five of seven animals of the heat-treated group were still alive at the end of experiment with no evidence of tumors.
Traditional hyperthermia methods are limited in the treatment of hepatic tumors. Many problems, such as over-heating of surface tissue and reflection from tissue-air interface, are often encountered with external microwave or ultrasound heating techniques. Interstitial technique, on the other hand, produces heat by means of directly implanting devices into the target area. Although invasive, interstitial source usually can localize heating in a tumor and minimize thermal damage to the surrounding normal tissues. Implanted electrodes and microwave antennas are used most frequently in interstitial techniques. However, the need for connecting the implanted devices to a power source and the invasive temperature monitoring limit the practice of these methods.
In recent years, thermoseed method for the brain tumors was reported in literatures[1-6]. The clinical trials described in tumor-bearing animals and patients have shown the thermoseed technique to be a promising method in tumor hyperthermia. In an effort to obtain orientated hyperthermia for hepatic tumors, we investigated the effects of thermoseed heating on subcutaneous hepatic tumors (H22 ) as the first step of our studies . This preliminary report suggests that the method is beneficial to heating selection and temperature control for treatment of hepatic tumor. When thermoseed is implanted into tumors, it can absorb energy from an externally applied electromagnetic induction field and act as a “seed in heating” within the tumors, thus make the localized heating of deepseated hepatic tumor possible. Automatic temperature regulation is another advantage in this method. Because of its lower Curie temperature, thermoseed has a function of self-regulating in the electromagnetic induction heating so as not to damage the surrounding normal tissues.
However, it is necessary to investigate how to warm relatively a large volume of tumor tissues before clinical applications. In our another experiment on rabbit (to be published), we found that when the renal artery was ligated during thermoseed hyperthermia, the temperature of the kidney increased by 10 °C. Obviously, the cooling effect by blood flow can not be ignored. Therefore, the combination therapy of hyperthermia and ligation or embolization of tumor supply artery may be beneficial to the treatment of large, deep-seated tumors.
Project supported in part by the Youth Medical Scientific Foundation of Chinese Ministry of Public Health, No.90207 and the Natural Scientific Foundation of Jiangsu Province, No.90058.
| 1. | Kobayashi T, Kida Y, Matsui M, Amemiya Y. [Interstitial hyperthermia of malignant brain tumors using implant heating system (IHS)]. No Shinkei Geka. 1990;18:247-252. [PubMed] |
| 2. | Mack CF, Stea B, Kittelson JM, Shimm DS, Sneed PK, Phillips TL, Swift PS, Luk K, Vora N, Stauffer PR. Interstitial thermoradiotherapy with ferromagnetic implants for locally advanced and recurrent neoplasms. Int J Radiat Oncol Biol Phys. 1993;27:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Brezovich IA, Atkinson WJ, Chakraborty DP. Temperature distributions in tumor models heated by self-regulating nickel-copper alloy thermoseeds. Med Phys. 1984;11:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Paulus JA, Richardson JS, Tucker RD, Park JB. Evaluation of inductively heated ferromagnetic alloy implants for therapeutic interstitial hyperthermia. IEEE Trans Biomed Eng. 1996;43:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Indik JH, Indik RA, Cetas TC. Fast and efficient computer modeling of ferromagnetic seed arrays of arbitrary orientation for hyperthermia treatment planning. Int J Radiat Oncol Biol Phys. 1994;30:653-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Chin RB, Stauffer PR. Treatment planning for ferromagnetic seed heating. Int J Radiat Oncol Biol Phys. 1991;21:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |