Published online Aug 15, 1998. doi: 10.3748/wjg.v4.i4.314
Revised: July 22, 1998
Accepted: August 4, 1998
Published online: August 15, 1998
AIM: To study the phase cancer tissue intercellular adhesion molecule-1 (ICAM-1) expression of human cancer metastasis model in nude mice, and to analyze the relationship between ICAM-1 expression and the metastasis and recurrence of hepatocellular cancinoma (HCC).
METHODS: HCC tissues from liver cancer metastasis model in nude mice (LCI-D20) was orthotopically implanted, and ICAM-1 expression in HCC tissues at different growing time were detected by immunodot blot. Tumor size, intrahepatic and extrahepatic metastasis foci were observed by naked eyes and under light microscope.
RESULTS: ICAM-1 was positively correlated to the tumor growing time (r = 0.88, P < 0.01) and tumor size r = 0.5, P < 0.05). It was higher in metastatic HCC than in nonmetastatic HCC (8.24 ± 0.95 vs 3.03 ± 0.51, P < 0.01). ICAM-1 content in cancer tissues increased suddenly after metastasis occurred and then maintained in a high level. ICAM-1 was also higher in multimetastasis group than in monometastasis group (10.05 ± 1.17 vs 5.48 ± 0.49, P < 0.05).
CONCLUSION: Tissue ICAM-1 could predict not only the metastasis of human liver cancer metastasis model in nude mice early and sensitively, but also the metastasis degree. So tissue ICAM-1 may be a potential index indicating the status of metastasis of HCC patients.
- Citation: Sun JJ, Zhou XD, Liu YK, Zhou G. Phase tissue intercellular adhesion molecule-1 expression in nude mice human liver cancer metastasis model. World J Gastroenterol 1998; 4(4): 314-316
- URL: https://www.wjgnet.com/1007-9327/full/v4/i4/314.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i4.314
Although many methods had been used to prohibit posthepatectomic metastasis of liver cancer in clinic, but the 5-year postoperative metastasis rate was still up to 61.9% in general, and 45.3% in small HCC[1]. Metastasis and recurrence have become the main obstacle in HCC patients to gain better outcome and longer survival. Up to now we can not diagnose or predict it before the formation of metastasis node. In the pervious studies, we found ICAM-1 was related to liver cancer and its metastasis, and tissue and serum ICAM-1 could predict the status of HCC metastasis[2,3]. It was unclear whether the ICAM-1 could reflect the HCC metastasis early and sensitively, and how ICAM-1 changed during the HCC growing time. So in this experiment, we observed the phase tissue ICAM-1 expression of human liver cancer high metastasis model in nude mice (LCI-D20) at different time period from tumor implantation to metastasis and telophase.
Monoclonal antibody of ICAM-1 (1 g/L) was purchased fromR & D Company, Britain. AKP-rabbit-anti-mouse IgG was from Sino-Amercan Company, China. NC membrane, NBT and BCIP were bought from Sigma Company.
BALB/cA male nude mice (Shanghai Institute of Materia Medica, Chinese Academy of Sciences, China) aged 4 to 6 weeks and the nude mice model of human hepatocellular carcinoma with high metastatic potential (LCI-D20)[4], which were established in our institute in 1996, were used in this study.
Tumor block of LCI-D20 nude mice human liver cancer metastasis model was implanted into the left lobe of the nude mouse liver according to the routine procedure in our laboratories. Briefly, a left upper abdominal transverse incision was made under anaesthesia; the left lobe of the liver was exposed and a part of the liver surface were mechanically injured with scissors. Then, a tumor block of 0. 2 cm × 0.2 cm × 0.2 cm was fixed within the liver tissue. After operation, mice were kept in laminar-flow cabinets under specific-pathogen-free conditions.
Mice were killed at day 7, 10, 13, 16, 19, 21, 25, 32 and 35 postimplantation, and tumor size, intrahepatic, lung, intra-abdominal metastasis nodes were found. The tumors were resected and frozen, and then stored at -70 °C for further use. The residual liver and lung were also resected and processed for routine gross and finding metastasis node under microscope.
Three hundredmg tissue was homogenized in 1.5 mL suspending buffer (0.1 mol/L NaCl, 0.01 mol/L Tris.Cl, pH 7.6, 0.001 mol/L EDTA pH 8.9, 1% Txiton-X100), the protein concentration was determined by Hartree method[5]. Thirty µl supernatant or 50 µl serum was applied onto the nitrocellulose membrane in a dot blot format, and physiological saline was used as control. After non-specific blocking with 5% lipid-free milk, the blots were incubated with ICAM-1 antibody (1:500) at room temperature for 2 h, followed by incubation with AKP-conjugated rabbit-anti-mouse-IgG (1:200) for 2 h at room temperature, then stained with NBT/BCIP (2:1v/v). The integrated optical density (IOD) of each blot was measured by MIAS-300 automatic image analyzer. Tissue ICAM-1 = [sample IOD-background IOD] × sample area/µg protein concentration/1000.
Student’s t test and linear correlation analysis were used statistically.
At the 7th day postimplantation, tumors were too small, only 0.2-0.3 in diameter, to take sample for ICAM-1 detection. At the 10th and 13 th day, tumor in only one mouse was big enough for ICAM-1 detection. No metastasis node was found before the 19th day. At the 19th day, metastasis nodes were found in 2 of the 3 killed mice, 1 with metastasis node in lung under optical microscope, 1 mouse with visible metastasis node in iliac fossa lymphnode. Later, metastasis nodes increased, which could be found in liver, lung, iliac fossa, inferior kidney, pelvic cavity, lymphnode of mesentery, para-aorta retroperitoneum, and at last bloody ascites.
| Tumor growing time (day) | Cases | ICAM-1 (-x±s) |
| 10 | 1 | 2.71 ± 0.00 |
| 13 | 1 | 2.78 ± 0.00 |
| 16 | 3 | 2.53 ± 0.53 |
| 19 | 3 | 5.30 ± 0.81 |
| 22 | 3 | 7.77 ± 1.21 |
| 32 | 4 | 8.69 ± 2.01 |
| 36 | 2 | 6.57 ± 1.95 |
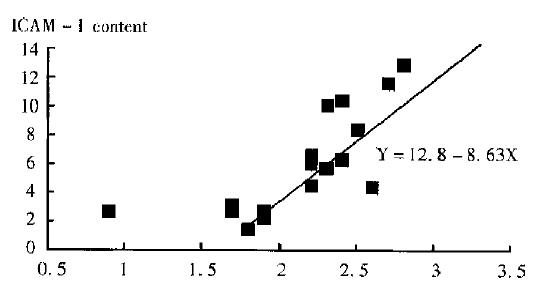
Tissue ICAM-1 was highly correlated to tumor growing time, the correlation coefficient(γ) was 0.88(P < 0.01). The time course of tissue ICAM-1 indicated that there was a plateau stage at day 10, 13 and 16 after implantation, then suddenly reached a higher level at day 19, meanwhile the metastatic node were discovered in lung and iliac fossa lymphnode. At this time ICAM-1 expression was nearly two times higher than that before day 19. Thereafter ICAM-1 expression in HCC continued and maintained in a high level until day 36. It became lower at day 36, probably because at this terminal HCC some necroses occurred inside the tumor. It was suggested that tissue ICAM-1 expression could act as an indicator of liver cancer metastasis in LCI-D20 model.
According to Figure 1, ICAM-1 increased as the tumor were growing larger. Statistical analysis revealed that ICAM-1 content in tissue was correlated to tumor size (γ = 0.5, P < 0.05). This suggested that tissue ICAM-1 could reflect the growing status of liver cancer.
Tissue ICAM-1 was significantly different between before metastasis (3.03 ± 0.51) and after metastasis (8.24 ± 0.95, P < 0.01), and between monosite metastasis (5.48 ± 0.49) and multisite metastasis (10.05 ± 1.17, P < 0.05). These data indicated that ICAM-1 might reflect not only the status but also the degree of the metastasis of LDI-20D liver cancer metastasis model.
The process of tumor metastasis is very complex, including the tumor cell dissociating from the primary locus, invading across the surrounding tissue, entering and extravasation from the circulation, and growing in distant organs[6]. Tumor angiogenesis, matrix degradation, cell adhesive molecule, oncogene, signal transduction, factors like IGF, ect are involved in this procedure[7]. Intercellular adhesion molecule-1 (ICAM-1), so called CD54, is a member of the immunoglobulin superfamily of adhesive molecule. It was found that ICAM related to cancer and cancer metastasis. Serum SICAM-1 (soluble ICAM-1) in patients with advanced stage (II-IV) or recurrent cervical uterine cancer increased significantly[8].
SICAM-1 was also related to lesion thickness, staging, recurrence probability of melonoma[9]. In lung cancer patients, SICAM-1 showed a significantly positive correlation with primary tumor size, and the overall survival of patients with low serum ICAM-1 concentration tend to be longer than that of patients with high serum ICAM-1 concentration[10]. Renal cancer recurrence was related to ICAM-1 expression in cancer tissues, patients with < 50% of ICAM-1 positive cell in cancer tissues showed improved disease-free survival after a median follow-up duration of 60 months [11]. But no study has been found on if serum or tissue ICAM-1 could indicate the early cancer metastasis and recurrence sensitively up to date according to our knowledge, neither the articles about phase change of ICAM-1 during tumor formation to tumor metastasis and terminal stage.
In this experiment, we analyzed the tissue ICAM-1 expression of LCI-D20 liver cancer metastasis model of different time after implantation, and found that tissue ICAM-1 expression was positively correlated to tumor size and tumor growing time. It is interesting that ICAM-1 content increased suddenly. It was also higher in HCC with multimetastasis nodes than in HCC with monometastasis node.
With this point of view, we conclude that tissue ICAM-1 could reflect both the metastasis of LCI-D20 human liver cancer metastasis model sensitively and the degree of metastasis early. ICAM-1 might be an index indicating the status of liver cancer metastasis clinically.
Project supported by Grant from Leading Specialty by the Shanghai Health Bureau, The Ninth 5-year Plan of National Medical Science and Technology 96-906-01-15, National Natural Science Foundation of China, No.396707 and China Medical Board, No. 93-583, New York, NY, USA.
| 1. | Zhou XD, Tang ZY, Yu YQ, Yang BH, Lu JZ, Lin ZY, Ma ZC, Zhang BH. Recurrence after resection of alpha-fetoprotein-positive hepatocellular carcinoma. J Cancer Res Clin Oncol. 1994;120:369-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Sun JJ, Zhou XD, Liu YK, Tang ZY. Study on the relationship between inter-cellular adhesion molecule-1 expression and the metastasis and recurrence of liver cancer. China Oncol. 1997;7:161-164. |
| 3. | Sun JJ, Zhou XD, Zhou G, Liu YK, Tang ZY. Serum intercellular adhesion molecule-1 and liver cancer metastasis and recurrence. Natl Med J China. 1998;4:383. |
| 4. | Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao DM, Ma ZC. Establishment of a metastatic model of human hepatocellular carcinoma in nude mice via orthotopic implantation of histologically intact tissues. Int J Cancer. 1996;66:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4025] [Cited by in RCA: 3697] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 6. | Saiki I, Iida J, Murata J, Ogawa R, Nishi N, Sugimura K, Tokura S, Azuma I. Inhibition of the metastasis of murine malignant melanoma by synthetic polymeric peptides containing core sequences of cell-adhesive molecules. Cancer Res. 1989;49:3815-3822. [PubMed] |
| 7. | Hart IR, Saini A. Biology of tumour metastasis. Lancet. 1992;339:1453-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 235] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Nasu K, Narahara H, Etoh Y, Kawano Y, Hirota Y, Miyakawa I. Serum levels of soluble intercellular adhesion molecule-1 (ICAM-1) and the expression of ICAM-1 mRNA in uterine cervical cancer. Gynecol Oncol. 1997;65:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Natali PG, Hamby CV, Felding-Habermann B, Liang B, Nicotra MR, Di Filippo F, Giannarelli D, Temponi M, Ferrone S. Clinical significance of alpha(v)beta3 integrin and intercellular adhesion molecule-1 expression in cutaneous malignant melanoma lesions. Cancer Res. 1997;57:1554-1560. [PubMed] |
| 10. | Osaki T, Mitsudomi T, Yoshida Y, Oyama T, Ohgami A, Nakanishi K, Nakanishi R, Sugio K, Yasumoto K. Increased levels of serum intercellular adhesion molecule-1 (ICAM-1) in patients with non-small cell lung cancer. Surg Oncol. 1996;5:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Santarosa M, Favaro D, Quaia M, Spada A, Sacco C, Talamini R, Galligioni E. Expression and release of intercellular adhesion molecule-1 in renal-cancer patients. Int J Cancer. 1995;62:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |