Published online Jun 15, 1998. doi: 10.3748/wjg.v4.i3.228
Revised: March 26, 1998
Accepted: May 5, 1998
Published online: June 15, 1998
AIM: To further study the role of bcl-2 protein expression in gastric carcinogenesis and tumor progression.
METHODS: Using immunohistochemical staining, the bcl-2 protein expression in 50 cases of gastric carcinoma and its relation to clinical status and pathomorphological parameters were observed.
RESULTS: Forty-one (82%) cases were positive for bcl-2 protein staining which was located in the cytoplasm and nuclear membrane of tumor cells. The rate of bcl-2 protein expression was not correlated with the patient, sex, tumor size, lymph node status or clinical stages (P > 0.05). It was strongly associated with intestinal-type tumors and poorly differentiated tumors (P < 0.05 and P < 0.01).
CONCLUSION: Aberrant bcl-2 protein expression appears to be specifically associated with development of intestinal-type gastric carcinoma, bcl-2 protein expression might play an important role in the early development/promotion and phenotypic differentiation of gastric carcinomas, but not in tumor progression.
- Citation: Liu HF, Liu WW, Fang DC, Men RP. Expression of bcl-2 protein in gastric carcinoma and its significance. World J Gastroenterol 1998; 4(3): 228-230
- URL: https://www.wjgnet.com/1007-9327/full/v4/i3/228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i3.228
Recently, emphasis has been placed on the role of apoptosis and its regulation in tissue homeostasis and carcinogenesis. To determine whether bcl-2 plays a role in the gastric carcinogenic sequence, an immunohistochemical study of bcl-2 expression in gastric carcinoma and its relation to clinical status, pathomorphological parameters was carried out.
Fifty cases of surgically resected gastric carcinomas (male 34, female 16; mean age 56.3 years) were extracted from the files of the Department of Pathology, Southwest Hospital, Third Military Medical University. All blocks were fixed in 10% formalin and embedded in paraffin. Serial sections were cut from each block in 4 μm, stained with hematoxylin and eosin and confirmed pathologically.
Immunohistochemical staining for bcl-2 protein was performed using SP technique with the following procedure: ① slides were diparaffinized in two changes of xylene for 10 min each and then were hydrated in decreasing concentrations of ethanol and rinsed in phosphate-buffered saline. Endogenous peroxidase was blocked by 3% H2O2 in methanol for 5 min, and then incubated for 10 min at room temperature in normal goat serum (1:20). ② slides were incubated with a 1:50 dilution of the primary rabbit antihuman bcl-2 monoclonal antibody (Santa Cruz, USA) for 30 min at 37 °C. A biotin-streptavidin detection system was employed with diaminobenzidine as the chromogen. ③ slides were washed twice with phosphate-buffered saline and incubated with the linking reagent (biotinylated anti-immunoglobulin) for 10 min at 37 °C. After rinsing in phosphate-buffered saline, the slides were incubated with the peroxidase-conjugated streptavidin label for 10 min at 37 °C, and incubated with diaminobenzidine and H2O2 for 10 min in the dark, the sections were then counterstained with hematoxylin. With each batch of test samples, a positive control consisting of a tissue section from tonsil was evaluated. In addition, a negative control was prepared for each sample using an irrelevant antibody of the same isotype as the primary antibody.
The immunostaining of bcl-2 was visually classified into four groups by observing 1000 tumor cells in areas of the sections: no staining present in any of tumor cells (-); slight staining in most of the tumor cells or less than 25% tumor cells with strong staining (+); 26%-50% tumor cells with strong staining (+ +); and strong staining in more than 51% tumor cells (+ + +). The classification was done by two senior pathologists who did not know the clinicopathological data.
Analysis of data was accomplished using Chi square test. P values less than 0.05 were considered to be statistically significant.
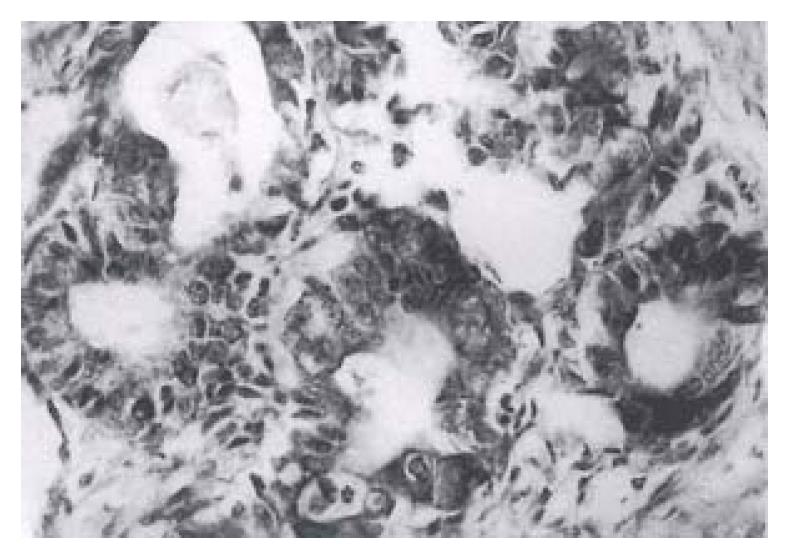
Forty-one (82%) of the fifty gastric carcinomas showed immunoreactivity for bcl protein in gastric carcinoma cells. Expression of varied: + in bcl-2 protein, 6 (12%), + + in 20 (40%) and + + + in 15 (30%). The bcl-2 protein immunoreactivity appeared brown or dark brown, which was localted on the cytoplasm and nuclear membrane of tumor cells (Figure 1). Some of the mature lymphocytes infiltrating in the stroma of gastric carcinomas had bcl-2 protein expression with a strong staining intensity.
Correlations between bcl-2 protein expression and clinical pathological data of gastric carcinoma are illustrated in Table 1. The rate of bcl-2 protein expression was not correlated with patient age, sex, tumor size, lymph node status and clinical stages (P > 0.05). The immunoreactivity of bcl-2 was significantly associated with morphologic phenotype and grades of differentiation of gastric carcinoma. Twenty-six (92.86%) of 28 gastric carcinomas of intestinal morphologic phenotype were immunoreactive versus 15 (68.16%) of 22 diffuse tumors (P < 0.05). Twenty-one (95.45%) of 22 poorly differentiated gastric carcinomas were immunoreactive versus 11 (61.11%) of 18 well and moderately differentiated carcinomas (P < 0.01).
| n | bcl-2 protein expression | Positive rate (%) | ||||
| - | + | + + | + + + | |||
| Age (a) | ||||||
| ≤ 59 | 36 | 7 | 4 | 15 | 10 | 80.56 |
| ≥ 60 | 14 | 2 | 2 | 5 | 5 | 85.71 |
| Sex | ||||||
| M | 34 | 7 | 2 | 13 | 12 | 79.41 |
| F | 16 | 2 | 4 | 7 | 3 | 87.50 |
| Type | ||||||
| Intestinal | 28 | 2 | 3 | 13 | 10 | 92.86a |
| Diffuse | 22 | 7 | 3 | 7 | 5 | 68.18 |
| Grade of differentiation | ||||||
| Well/moderate | 18 | 7 | 1 | 5 | 5 | 61.11 |
| Poor | 22 | 1 | 4 | 12 | 5 | 95.45b |
| Mucoid | 10 | 1 | 1 | 3 | 5 | 90.00 |
| Tumor size | ||||||
| < 5 cm | 31 | 8 | 4 | 11 | 8 | 74.19 |
| ≥ 5 cm | 19 | 1 | 2 | 9 | 7 | 94.74 |
| Lymph-node metastasis | ||||||
| Negative | 21 | 4 | 2 | 9 | 6 | 80.95 |
| Positive | 29 | 5 | 4 | 11 | 9 | 82.76 |
| Serosal invasion | ||||||
| Absent | 25 | 7 | 3 | 9 | 6 | 72.00 |
| Present | 25 | 2 | 3 | 11 | 9 | 92.00 |
| Clinical stages | ||||||
| I and II | 32 | 6 | 5 | 12 | 9 | 81.25 |
| III and IV | 18 | 3 | 1 | 8 | 6 | 83.33 |
The bcl-2 proto-oncogene is initially identified in human follicular and diffuse B-cell lymphomas characterized by the reciprocal translocation[1]. The product of the proto-oncogene bcl-2 is a 26-kDa protein that blocks apoptosis. bcl-2 protein is localized in the mitochondria, endoplasmic reticulum and nuclear envelope membranes. More recently, several normal and malignant tissues other than hematolymphoid cells have been shown to express bcl-2, including the lung, breast, prostate, stomach, small bowel and colon[2-5].
The relation of bcl-2 protein expression to clinicopathological parameters of tumors was still unclear. Joensuu et al[6] demonstrated that bcl-2 expression was correlated with the age of patients, which was more common in aged than in young patients. Silviastrini et al[7] found that bcl-2 protein expression was related to the tumor size. A significantly higher fraction of bcl-2 positive cells was observed in small tumors than in large tumors. Sierra et al[8] found that bcl-2 protein was more frequently expressed in tumors with early metastasis than in lymph-node negative tumors. Our study showed that bcl-2 protein expression was associated with morphologic phenotype and grades of differentiation of gastric carcinomas. The difference in the bcl-2 protein expression in the intestinal and diffuse types demonstrated that aberrant bcl-2 protein expression was preferentially associated with development of intestinal-type gastric carcinoma, indicating again the different biologic mechanisms involved in the development of these two histologic subtypes. The difference in the bcl-2 protein expression between poorly differentiated and well/moderately-differentiated gastric carcinomas demonstrated that aberrant bcl-2 protein expression was associated with differentiation or growth speed of gastric carcinomas. There was no significant relationship between bcl-2 protein expression and tumor size, lymph-node metastasis, serosal invasion or clinical stages. Therefore, bcl-2 protein expression might play an important role in the early development and phenotypic differentiation of gastric carcinomas, but not so in tumor progression.
Key project of the 9th 5-year plan for Medicine and Heath of Army, No. 96Z047.
| 1. | Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879-886. [PubMed] |
| 2. | Higashiyama M, Doi O, Kodama K, Yokouchi H, Tateishi R. High prevalence of bcl-2 oncoprotein expression in small cell lung cancer. Anticancer Res. 1995;15:503-505. [PubMed] |
| 3. | Lauwers GY, Scott GV, Karpeh MS. Immunohistochemical evaluation of bcl-2 protein expression in gastric adenocarcinomas. Cancer. 1995;75:2209-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Alderson LM, Castleberg RL, Harsh GR, Louis DN, Henson JW. Human gliomas with wild-type p53 express bcl-2. Cancer Res. 1995;55:999-1001. [PubMed] |
| 5. | Lu QL, Poulsom R, Wong L, Hanby AM. Bcl-2 expression in adult and embryonic non-haematopoietic tissues. J Pathol. 1993;169:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Joensuu H, Pylkkänen L, Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 1994;145:1191-1198. [PubMed] |
| 7. | Silvestrini R, Veneroni S, Daidone MG, Benini E, Boracchi P, Mezzetti M, Di Fronzo G, Rilke F, Veronesi U. The Bcl-2 protein: a prognostic indicator strongly related to p53 protein in lymph node-negative breast cancer patients. J Natl Cancer Inst. 1994;86:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 321] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Sierra A, Lloveras B, Castellsagué X, Moreno L, García-Ramirez M, Fabra A. Bcl-2 expression is associated with lymph node metastasis in human ductal breast carcinoma. Int J Cancer. 1995;60:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |