Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.144
Revised: December 30, 1997
Accepted: January 10, 1998
Published online: April 15, 1998
AIM: To determine the site of production and uptake of tumor necrotic factor alpha (TNFα), and evaluate the relationship between serum TNFα and plasma endotoxin (ET) in rats with acute hemor-rhagic necrotic pancreatitis (AHNP).
METHODS: Sprague Dawley rats were divided into AHNP group and control group (n = 12). AHNP model was induced by retrograde injection of 5% sodium taurocholate via pancreatic bile duct. The blood samples were obtained through portal vein 2 and 6 h after the operation.
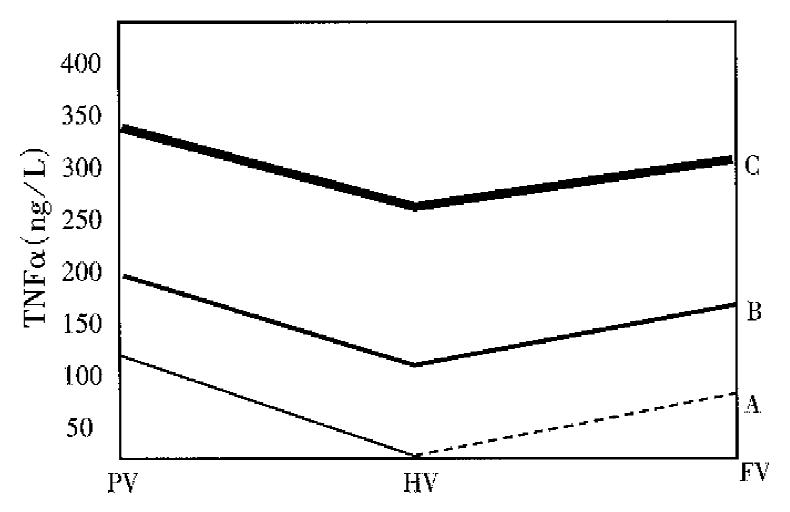
RESULTS: The contents of TNFα in portal vein were increased rapidly in the development of AHNP. They were lower in hepatic vein (280.59 ± 20.02) and femoral artery (310.82 ± 7.97) than in portal vein (354.91 ± 25.50) (P < 0.05), and higher in femoral artery than in hepatic vein 6 h after the operation (P < 0.05). TNFα level in plasma was increased significantly when ET level in portal vein showed no increase.
CONCLUSION: Pancreas, spleen, liver, intestinal tract and lung are the main organs to produce TNFα, and liver is also an important site for TNFα uptake in the development of AHNP. Plasma endotoxin is not a trigger for TNFα release in rats with AHNP.
- Citation: Qin RY, Zou SQ, Wu ZD, Qiu FZ. Experimental research on production and uptake sites of TNFα in rats with acute hemorrhagic necrotic pancreatitis. World J Gastroenterol 1998; 4(2): 144-146
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/144.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.144
Recent studies have indicated that during the pathogenesis of acute hemorrhagic necrotic pancreatitis (AHNP), tumor necrotic factor alpha (TNFα) and endotoxemia play important roles in the progression of pancreatic inflammation and the damage of expancreatic vital organs£Û1£Ý. The purpose of this paper is to determine the production and uptake sites of TNFα, and the relationship between TNFα and plasma endotoxin (ET) in rats with AHNP, thus providing a direct theoretical basis for the treatment of AHNP by blocking the production of TNFα and clearing the secreted TNFα in plasma.
Twenty-four Sprague-Dawley (SD) rats weighing 295 g-320 g were divided randomly into AHNP group and control group (12 animals for each group).
SD rats were fasted for 24 h, and were anesthetized by intraperitoneal injection of sodium pantobarbital (30 mg/kg). Normal saline (0.9%) was injected into each rat through a PE-50 placed catheter in femoral vein. Sodium taurocholate (5% 1.5 mL/kg) were infused retrogradely to AHNP rats through pancreatic bile duct under a microsurgical procedure, after 5 min-10 min, pancreatic edema and dotted bleeding occurred. Isovolume of 0.9% normal solution was infused to the pancreatic bile duct of control rats using the same method, and no abnormality was noticed 2 and 6 h after the operation. Blood samples from hepatic vein, portal vein and femoral artery were harvested, at these time points, hemorrhage, necrosis of pancreas and hydremic ascites (5 mL-10 mL) were found in AHNP-rats, while no abnomality was seen in the control rats.
TNFα in portal vein, hepatic vein and femoral artery were determined with ELISA (provided by the Department of Immunology, the Third Military Medical University). ET in portal vein was assayed by a quantitative azostromatic coloration limulus test (kit provided by the Shanghai Medical Analysis Institute).
| Groups | Time (h) | PV | HV | RHU (%) | FA |
| Control | 2 | 54.83 ± 3.24 | |||
| 6 | 52.14 ± 1.87 | ||||
| AHNP | 2 | 182.48 ± 9.90 | 119.85 ± 5.64 | 38.63 | 127.32 ± 7.15 |
| 6 | 354.91 ± 25.50 | 280.59 ± 20.02 | 20.95 | 310.82 ± 7.97 |
There was significant difference in TNFα levels among the samples from portal vein, hepatic vein and femoral artery (P < 0.05) 2 and 6 h after operation, and the difference was also significant as compared with the control group (P < 0.05). Two hours after operation, TNFα level in hepatic vein and femoral artery was lower than in portal vein (P < 0.05), but no difference was observed between that in femoral artery and hepatic vein. Six hours after operation, TNFα level in femoral artery was obviously higher than in hepatic vein (P < 0.05), but remained lower than in portal vein (P < 0.05). There was significant difference in the rate of hepatic uptake of TNFα between AHNP group and control group (P < 0.05).
| Groups | Time (h) | Plasma ET |
| Control | 2 | 0.022 ± 0.007 |
| 6 | 0.033 ± 0.006 | |
| AHNP | 2 | 0.028 ± 0.002 |
| 6 | 0.340 ± 0.038 |
Two and 6 hours after operation, there was significant difference in plasma ET level in portal vein of AHNP rats. When compared with the control group, significant difference of plasma ET level in portal vein of AHNP rats was observed at 6 hours, but not at 2 h after operation.
TNFα is an important inflammatory mediator during the development of AHNP. It can damage the pancreas and ex-pancreatic vital organ through the following mechanism: ① Enhancing the adhesion of PMN to endothelium. Recent research shows that there are some adhesive molecules or receptors, such as ICAM-1, ELAM-1 and VCAM-1 on the surface endothelia. TNFα can stimulate the expression of receptors for adhesive molecules on the surface of endothelium, leading to the adhesion of unstimulated PMN to endothelia and the migration of the PMN to inflammatory loci through vascular barrier. The interaction of PMN with target tissue cells and the releasing of oxygen free radicals (OFR) and neutral proteinase could cause the damage of adjacent tissues. ② The cytotoxic effect of TNFα. TNFα can bind with the specific receptors on the cellular membrane and facilitate the production of OFR, which damages the cell membrane and DNA, and cleaves DNA into fragments by endonuclease, leading to the death of cells. ③ TNFα can activate the inflammatory cells, stimulate the release of PMN from bone marrow, and promote the degranulation, the production of OFR and the releasing of lyso-some enzyme, alkali proteinase, etc. from eosinocytes. The persistent increase of plasma TNFα may promote the endocytosis, degranulation and the releasing of OFR and leukocyte enzymes (lysosome enzyme, plastic enzyme, etc.), resulting in the continuous hemorrhage, necrosis of pancreas and the development of MOF.
The exact mechanism of production, the production and absorption site of TNFα during the progression of AHNP remained unknown[2]. The stimulation of inflammatory cells by pancreatic enzyme was believed to be related with the production of TNFα . It is indicated that the disturbance of microcirculation of pancreas may lead to release of trypsinogen activated peptides[3], suggesting that the disturbance of microcirculation of pancreas may be the primary factor responsible forproduction of TNFα. Our results revealed that, at the early stage of AHNP, the TNFα level in portal vein was elevated obviously, which was significantly higher than in hepatic vein and femoral artery. There was no difference in TNFα level between hepatic vein and femoral artery. So it is concluded that at the early stage of AHNP, TNFα in portal vein may originate from pancreas, spleen and gastrointestinal tract, and the liver may clear some of TNFα originated from portal vein. With the aggravation of AHNP, TNFα in portal vein increased rapidly, and so did that in femoral artery, both being higher than in hepatic vein, but still lower than in portal vein. Based on these results, we believe that lung may be the organ of secondary production site of TNFα. TNFα in hepatic vein mainly originate from pancreas, spleen and gastrointestinal tract, and part of TNFα in hepatic vein might come from the liver. Although the liver could clear some TNFα , its potential is somewhat limited. As the hepatic damage gets worse, its clearance to TNFα decreases.
TNFα is a soluble polypeptide with an MW of 17 Ku, under the condition of severe infection, macrophagocytes, T cells and NK cells would produce TNFα due to the action of Gram negative bacteria and lipopolysaccharide. It is believed that at early stage of AHNP, the production of TNFα is not associated with ET[4]. Our results showed that plasma TNFα level elevated significantly when there was no increase of ET level in portal vein, indicating that ET was not the primary factor for the production of TNFα in AHNP rats, but TNFα level elevated much more significantly with the increase of ET. Recent studies also showed that, in late-stage AHNP, ET was contributed to the substantially in-creased production of TNFα. The synergetic effect of TNFα and ET is an important cause of high morbidity and mortality of AHNP. Therefore, control of infection, inhibition of the TNFα production, and removal of secreted TNFα are of great importance for the treatment of AHNP.
Project supported by the China Postdoctoral Sciences Foundation, No.C.P.S.F. 1996.2#.
| 1. | Hughes CB, Grewal HP, Gaber LW, Kotb M, El-din AB, Mann L, Gaber AO. Anti-TNFalpha therapy improves survival and ameliorates the pathophysiologic sequelae in acute pancreatitis in the rat. Am J Surg. 1996;171:274-280. [PubMed] |
| 2. | Grewal HP, Kotb M, el Din AM, Ohman M, Salem A, Gaber L, Gaber AO. Induction of tumor necrosis factor in severe acute pancreatitis and its subsequent reduction after hepatic passage. Surgery. 1994;115:213-221. [PubMed] |