Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.137
Revised: January 22, 1998
Accepted: March 15, 1998
Published online: April 15, 1998
AIMS: To study the regulatory effects of bacterial lipopolysaccharide (LPS) in murine macrophage proliferation.
METHODS: Using murine peritoneal exudate macrophage (PEM) and macrophage cell line J774A.1 as targets, LPS effects on M-CSF and granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulated macrophage colony-forming cells (CFU-M) were detected. 125I-GM-CSF receptor binding assay was used to examine LPS regulation on GM-CSF receptor expression. RT-PCR was employed to test TGF-β1 inhibition on IFN-γ mRNA expression on macrophage induced by LPS.
RESULTS: Without direct effect on macrophage proliferation, LPS could inhibit the macrophage proliferation stimulated by GM-CSF. However, with the concomitant existence of GM-CSF and TGF-β1, the LPS inhibitory effect was eliminated. RT-PCR analysis indicated that the strongest macrophage growth inhibitory factor IFN-γ mRNA expression in macrophage induced by LPS was remarkably sup-pressed by TGF-β1, 125I-GM-CSF receptor binding assay showed that LPS could enhance GM-CSF receptor expression likewise as TGF-β1.
CONCLUSIONS: LPS is involved in the network of macrophage proliferative regulation by multiple cytokines, displaying inhibitory and stimulatory effects based on the coexisting cytokines.
- Citation: Fan K. Regulatory effects of lipopolysaccharide in murine macrophage proliferation. World J Gastroenterol 1998; 4(2): 137-139
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.137
Lipopolysaccharide (LPS), main component of Gramª² negative bacterial endotoxin, is involved in immune reaction by intriguing macrophage and B lymphocyte. It has been demonstrated that interferon-γ (IFN-γ) is the strongest inhibitor of macrophage colony formation[1,2,6]. Therefore, LPS is thought to inhibit macrophage growth. Recent reports indicated that LPS at low concentration (< 0.1 µg/L) had stimulatory effects in some hematopoietic cells[3,4]. So, the effects of LPS in cell growth regulation is multifunctional. Tissue macrophges migrated from peripheral monocytes are the main targets of LPS and participate in immune response by LPS activation. Besides, macrophages are known to be able to form colony under M-GSF and GM-CSF stimulations. In this study, LPS effects in macrophage proliferation was investigated.
Murine peritoneal exudate macrophages (PEM) in 6-8-week old CsH/HeJ mice were induced by thioglocallate medium J774A.1 was obtained from ATCC. Reagent rMuG-M-CSF was obtained from Immunex Co, rHum-M-CSF as gift from Cetus Co, rHuTGF-β1 from NCI, and LPS was purchased from List Co, and RT-PCR Kit and primers from Cetus Co. GM-CSF and M-CSF iondination Bolton-Hunter method was used for GM-CSF iondination and chlormate T method for M-CSF iondination. Receptor binding assay was undertaken according to Reference 5.
PEM 1000/mL in a medium containing 10% FCS (Hyclone Co.) was cultured in 24-well plates at 5% CO2 atomasphere and 37 °C temperature. rMuGM-CSF (0.5 µg/L), LPS (0.01 µg/L-100 µg/L) or rHuTGF-β1 (0.5 µg/L) were added at different intervals. After 7-9 d, CFU-M (cell number > 50) was formed and calculated by a Coulter counter.
Reverse-transcription polymerase chain reaction (RT-PCR) was applied to examine cytokine mRNA expression. Experimental detail was referred to Reference 5. The IFN-γ and β-actin primers are as follows: IFN-γ 5’-primer(71): TGAACGCTACACACTGCTTCTTGG3’primer(530): CGACTCCCTTTTCCGCTTCCTAG β-actin 5’-primer(25): GTGGGCCGCTCTAGGCACCAA3’-primer (564): CTCTTTGATGTCACGCACGATTTC
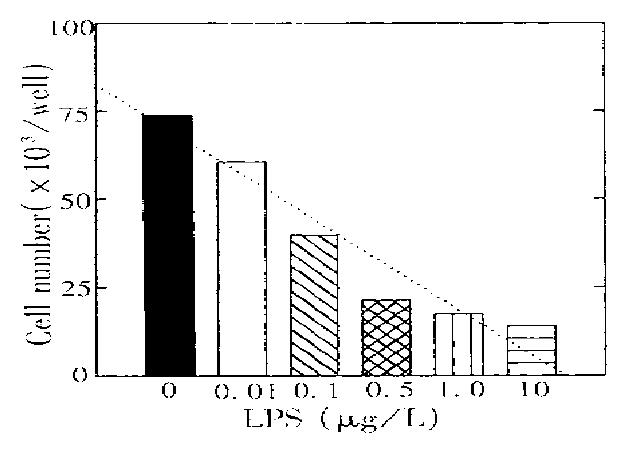
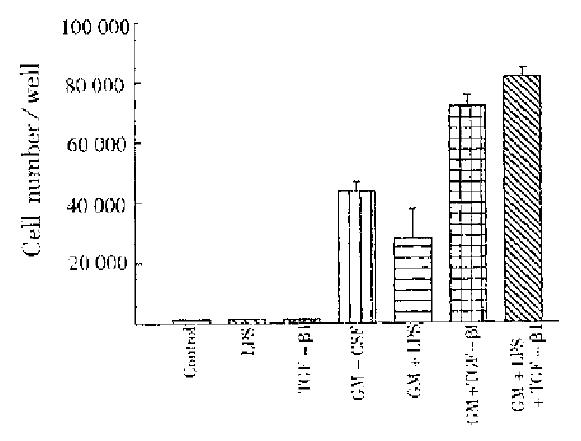
PEM was potential to form CFU-M in vitro under optimal GM-CSF stimulation. Figure 1 shows that LPS at a concentration of lower than 1.0 μg/L considerably inhibited macrophage growth and this inhibition was typically dose-dependent. However, with concomitant addition of rHuTGF-β1 (0.5 μg/L), LPS inhibition was reversed to be stimulatory while LPS and TGF-β1 had no direct effects in CFU-M (Figure 2). Our previous paper reported that TGF-β1 enhancement on GM-CSF stimulation was associated with the increase of GM-CSF receptor number[6]. Accordingly, LPS effect in GM-CSF receptor regulation was further examined.
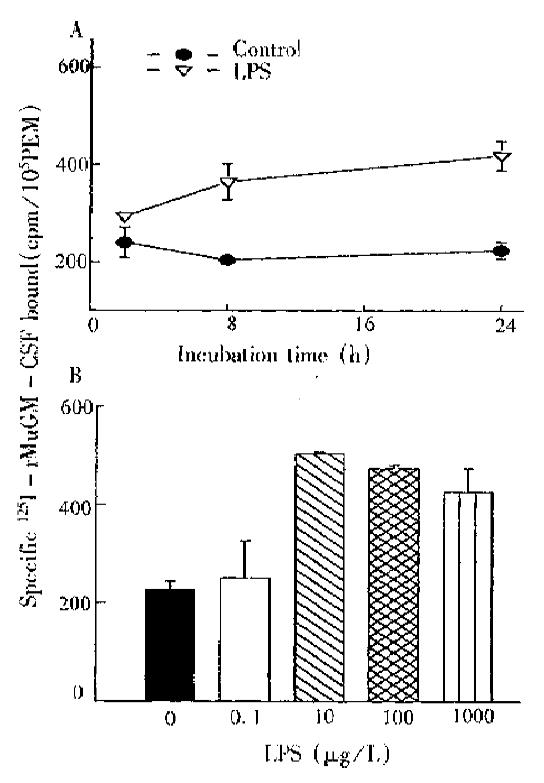
LPS could remarkably decrease M-CSF receptor number within a short time (37 °C, 1 h), but without effect on GM-CSF. After LPS treatment in PEM was prolonged to 24 h, GM-CSF receptor number was increased by over 80%, being time-dose dependent (Figure 3).
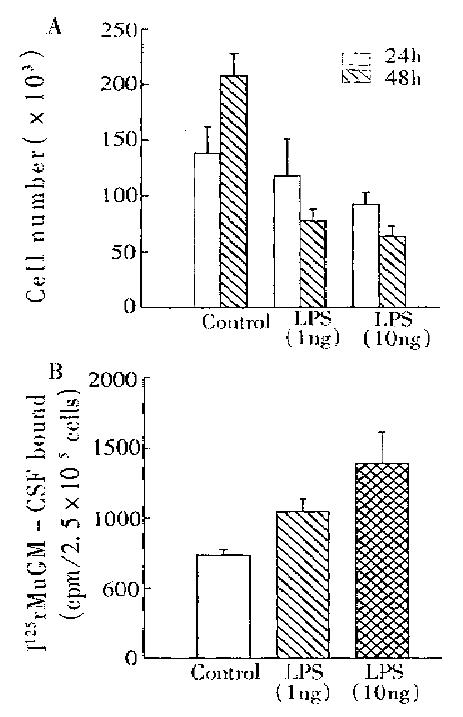
Although murine macrophage cell line J774A.1 autonomous growth was inhibited by LPS, its membrane GM-CSF receptor number was enhanced (Figure 4). These results confirmed the upregulation of LPS in GM-CSF receptor expression.
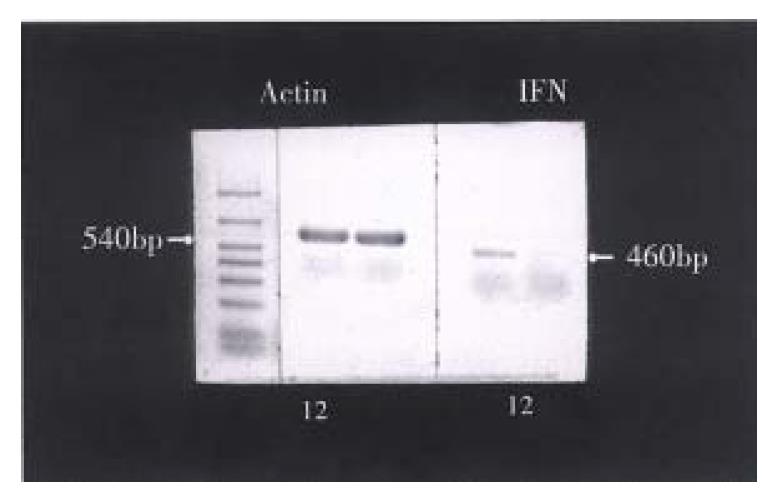
IFN-γ mRNA expression in PEM was examined with (RT-PCR) (Figure 5). Consequently, IFN-γ mRNA was expressed after LPS induction in PEM for 2 h, which was totally suppressed by TGF-β1 treatment for 2 h.
Macrophages play an important role in a series of physiological and pathogenic processes, such as antiinfection, anti-tumor, immune response and inflammation. Macrophage growth regulation is modulated by multiple cytokines. Among them only GM-CSF and M-CSF can stimulate macrophage colony formation. Others (IL-4, TNF-α, L-3, IFN-γ, TGF-β1) influence macrophage growth by regulating M-CSF or GM-CSF receptor number and expression[4-7]. Bacterial lipopolysaccharide mainly activates macrophage and plays a certain role in its proliferation. LPS at in vivo concentration of 0.1 μg/L-10 μg/L, can inhibit GM-CSF stimulated macrophage growth but without direct effect. Interestingly, the addition of TGF-β1 turns the LPS effect from inhibitory to stimulatory. Receptor binding assay indicates that LPS and TGF-β1 can both enhance GM-CSF receptor number, probably because of effects of the three modulators (GM-CSF, LPS and TGF-β1) in macrophage growth. As IFN-γ is induced by LPS and is the strongest autocrine inhibitor of macrophage growth. TGF-β1 may suppress IFN-γ expression in macrophage by RT-PCR technique. According to our results, a regulating network of LPS in macrophage proliferation is presumed. LPS is capable of increasing GM-CSF receptor number, but IFN-γ displays stronger inhibition on macrophage growth. With the coexitence of TGF-β1, IFN-γ expression is remarkably suppressed and GM-CSF receptor number is further enhanced. As a result, the concomitant presence of LPS, TGF-β1 and GM-CSF stimulates macrophage proliferation. Our findings could lead to a conclusion that it is necessary to further reveal the complexity, complementarity and continuity of three or more cytokines in macrophage proliferation.
| 1. | Rennick D, Yang G, Gemmell L, Lee F. Control of hemopoiesis by a bone marrow stromal cell clone: lipopolysaccharide- and interleukin-1-inducible production of colony-stimulating factors. Blood. 1987;69:682-691. [PubMed] |
| 2. | Ding AH, Nathan CF. Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J Immunol. 1987;139:1971-1977. [PubMed] |
| 3. | Moore RN, Steeg PS, Männel DN, Mergenhagen SE. Role of lipopolysaccharide in regulating colony-stimulating factor-dependent macrophage proliferation in vitro. Infect Immun. 1980;30:797-804. [PubMed] |
| 5. | Fan K, Ruan Q, Sensenbrenner L, Chen BD. Up-regulation of granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors in murine peritoneal exudate macrophages by both GM-CSF and IL-3. J Immunol. 1992;149:96-102. [PubMed] |
| 6. | Chen BD, Chou TH, Ratanatharathorn V. Expression of gamma-interferon receptor in murine bone marrow-derived macrophages associated with macrophage differentiation: evidence of gamma-interferon receptors in the regulation of macrophage proliferation. J Cell Physiol. 1987;133:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |