Published online Apr 15, 1998. doi: 10.3748/wjg.v4.i2.103
Revised: December 26, 1997
Accepted: February 24, 1998
Published online: April 15, 1998
AIM: To observe the effects of a chemically synthesized tetrose and a natural yeast mannan on experimental liver metastasis of mouse melanoma.
METHODS: After treated with 4mg tetrose (tetrose group) or 4 mg mannan (mannan group) for 30 min at 37 °C, 0.5 ml 1 × 106 B16-MBK melanoma cells were injected into the spleen of mice. Fifty-five days later, melanoma metastatic nodes on the surface of the liver and in other organs as well as mouse survival time were observed.
RESULTS: Of the 6 mice in control (B16 cell + PBS) group, 4 died naturally within 55 d, and 2 were killed on the 55th day. All of the 6 mice had metastases in livers, the total number of the melanoma nodes on each liver surface ranged from 2 to 30, with the largest one merging into the whole liver. One mouse had a neo-plasm in the remnant site of injection, and 3 had metastases in lungs. In contrast, of the 6 mice in tetrose group, only one died on the 50th day after injection, with 3 metastases in the liver, the largest being 10 mm in diameter, the other 5 mice survived until being dissected on the 55th day after injection and had no liver metastasis, but 3 of them had neoplasms in their remnant sites of injection. In mannan group, all of the 6 mice survived and no metastasis was seen except for 2 liver nodes in one mouse with the largest diameter of 1 mm. Neither tetrose nor mannan group had metastasis out of the liver, and the weight of liver in the two groups was significantly lower than those in the control group.
CONCLUSION: Both tetrose and mannan had the effects of preventing melanoma cells from experimental metastasis to and out of the liver, and prolonging the survival time of the mouse.
- Citation: Liu YP, Zhou RL, Wang YF, Cai MC. Inhibitory effects of two oligosaccharides on murine melanoma experimental liver metastasis. World J Gastroenterol 1998; 4(2): 103-105
- URL: https://www.wjgnet.com/1007-9327/full/v4/i2/103.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i2.103
The saccharide structures on the surface of tumor cells, particularly of the highly metastatic cancer cells, have certain peculiarities, and may play vital roles in the process of metastasis. We have demonstrated that some glycopeptides can significantly inhibit experimental metastasis of mouse melanoma and Lewis lung carcinoma cell lines towards the lungs and livers[1-3], and the antimetastatic effects of the glycopeptides lie in their carbohydrate moieties. In order to explore the structural peculiarity associated with the metastasis-blocking function, and to develop new antimetastatic drugs, we designed and chemically synthesized a tetrose with a special structure, and chose the natural mannan to compare the effects of carbohydrate structures on experimental liver metastasis of mouse melanoma cells.
Eighteen male Balb/c mice with similar body weight, obtained from the Animal Center of Beijing Medical University, were divided randomly into control group, tetrose group and mannan group, respectively, with 6 mice in each group. B16-MBK melanoma cell line (B16 cell for abbreviation) was provided by the Department of Cell Biology, Basic Medical Research Institute of Chinese Academy of Medical Sciences. Chemically synthesized tetrose was produced by the Department of Organic Chemistry in Beijing Medical University. RPMI 1640 was provided by JR Scientific Company, USA. Other reagents were A.R. or C.P. grade made domestically.
Induction of liver metastases. Lafremiere’s method was adopted[4]. Briefly, with the use of sterile instruments and gloves, Balb/c mice, after anaesthetized by ether inhalation, were set in the right lateral position, and a 1-cm incision was made at the left subcostal region. The spleen was gently retracted, and the short gastric vessels and gastros-plenic ligament at the upper pole of the spleen were identified, ligated and cut, thus freeing the spleen at its pedicle. This procedure allowed the spleen to be exposed outside the abdominal cavity. B16 cell suspension 0.5 mL (2 × 106 cells/mL) in serum-free 1640 medium was injected through a 27-gauge needle positioned in the spleen through its upper pole. The spleen pedicle was clipped with a medium hemoclip, then the spleen was removed, and the spleen pedicle repositioned intraperitoneally. The abdominal cavity was closed in one layer. The animals were fed with standard mouse chow and water ad libitum. Fifty-five days later, the mice were killed and their livers were harvested, the tumor metastasis nodes were counted on the surface of the livers.
Fourty-eight hours after passage culture, the B16 cells were detached by brief digestion with 0.125% trypsin (in PBS without Ca and Mg), the digestion was stopped by 1640 medium with 10% fetal bovine serum and then the cells were resuspended in serum-free 1640 medium, readjusted to the cell concentration at 2 × 106/mL. After incubated respectively with 4 mg tetrose (tetrose group) or 4 mg mannan (mannan group) for 30 min at 37 °C in a humidified air atmosphere of 5% CO2, the cell viability was assessed > 95% by 0.2% trypan blue exclusion test, and the cells were injected as described above in the spleen. In the control group, the cells were treated with PBS substitutionally.
The significance of differences among groups was determined by the Student’s t test or χ2 test. Two-tail P values were presented for all experiments.
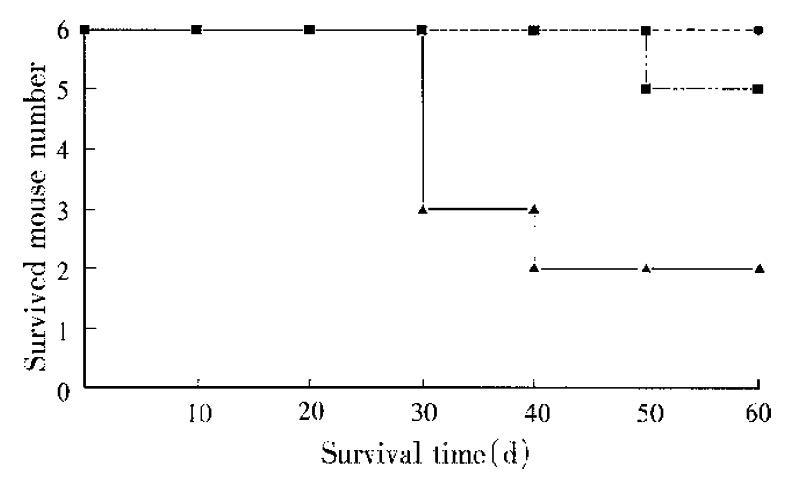
With 1 × 106 cells injected intraspleen, 4 mice in the control group died naturally within 55 d after the injection of B16 cells, and 2 mice were killed on the 55th day (Figure 1). All the mice in the control group had metastases in the livers, the number of the metastastic nodes ranged from 2 to 30, with the largest one merged into the whole liver. The liver weights were 3.8 g ± 1.5 g. Of the 6 mice, 3 had lung metastases simultaneously and one mouse was found to have a neoplasm in the injected area.
Tetrose group. One of the 6 mice died on the 50th day of injection with 3 melanoma nodes on its liver surfaces, the largest diameter was 10 mm, the other 5 were killed on the 55th day, 3 of which had neoplasms in their remnant sites of injection, but without metastases either in or out of the livers. The weights of livers (1.4 g ± 0.1 g) were significantly lower than the control group (Table 1).
Mannan group. All of the 6 mice survived until the dissection day, and only one mouse had 2 metastases in the liver with the node diameters < 1mm, others had no metastases either in or out of the livers. The livers weighted 0.85 g ± 0.02 g, being significantly lower than the control (P < 0.05).
In our experimental liver metastasis model, intrasplenic injection of B16 cells was used to induce the cells into portal circulation, and allow the tumor cells to form liver metastases. The fact that all mice in the control group developed metastases in the livers indicated that the model was highly reproducible, carried a high metastatic rate, and therefore was a preferable model for experimental liver metastasis. Complete ligation of the gastric and other vessels and prevention of direct spleen clamping were critical, and leakage should be avoided, if it appears, the mouse should be given up.
Recently, Dean et al[5] successfully inhibited experimental metastasis to lungs using synthetic multivalent lactosyl clusters. Tsukada et al[6] proved the antimetastatic and growth inhibitory effects of N-acetylchitohexaose in mouse Lewis lung carcinoma. The tetrose and mannan in the present study, with the structure differing from the above two carbohydrates, are strikingly effective in inhibiting experimental liver metastases. The tetrose we designed exists in the saccharide chains of tumor cell surface, its action suggested that the carbohydrate with this structure may block the metastatic process of tumor cells. The antimetastatic effect of mannan is also inspiring. Chandrasekaran et al[7] demonstrated in vitro that the spreading of B16 cells on basement membrane depends on the N-linked high mannose carbohydrate structure. The present study showed that after incubated with mannan, the B16 cells were blocked to metastasize toward the liver, probably by the blocking effect of mannan on the interaction, including spreading of B16 cells and basement membrane. As we know, spreading is a prerequisite of a series of biological processes (such as secreting proteinase, migration and proliferation) in invasion of tumor cells. It should be noted, however, that the tetrose suppressed experimental liver metastases without inhibiting the neoplasia, since there were neoplasms in the remnant sites of injection in some mice in the tetrose group. The findings also proved that the antimetastatic effect was not due to the cytotoxic effect of the oligosaccharides, as all cells were fully viable after the treatment with tetrose or mannan. All these demonstrated that these two oligosaccharides selectively inhibited the experimental meta-stasis of mouse melanoma cells.
Project supported by the National Natural Science Foundation of China, No.39370169.
| 1. | Zhao Y, Zhou RL. The anti-metastatic effect of laminin glycopeptides in mouse B16-MBK melanoma experimental metastasis. J Beijing Med Univ. 1992;24:404-406. |
| 2. | Zhang QY, Zhou RL, Zhang CY. The pathological observation of anti-metastatic effect of glycopeptides in mouse Lewis lung carcinoma experimental metastasis. J Biochem. 1991;7:719-721. |
| 3. | Liu YP, Zhou RL, Zhang S. Laminin glycopeptides inhibit mouse melanoma liver metastasis. J Beijing Med Univ. 1996;28:91-92. |
| 4. | Lafreniere R, Rosenberg SA. A novel approach to the generation and identification of experimental hepatic metastases in a murine model. J Natl Cancer Inst. 1986;76:309-322. [PubMed] |
| 5. | Dean B, Oguchi H, Cai S, Otsuji E, Tashiro K, Hakomori S, Toyokuni T. Synthesis of multivalent beta-lactosyl clusters as potential tumor metastasis inhibitors. Carbohydr Res. 1993;245:175-192. [PubMed] |
| 6. | Tsukada K, Matsumoto T, Aizawa K, Tokoro A, Naruse R, Suzuki S, Suzuki M. Antimetastatic and growth-inhibitory effects of N-acetylchitohexaose in mice bearing Lewis lung carcinoma. Jpn J Cancer Res. 1990;81:259-265. [PubMed] |
| 7. | Chandrasekaran S, Tanzer ML, Giniger MS. Oligomannosides initiate cell spreading of laminin-adherent murine melanoma cells. J Biol Chem. 1994;269:3356-3366. [PubMed] |