Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.48
Revised: July 16, 1997
Accepted: August 23, 1997
Published online: February 15, 1998
AIM: To investigate the expression of somatostatin mRNA in various differentiated types of gastric carcinoma.
METHODS: By using in situ hybridization and immunohistochemical techniques, the expression of somatostatin mRNA and somatostatin immunoreactivity in the normal gastric mucosa, the poorly, moderately and well-differentiated gastric carcinomas, and various clinical stages of carcinoma were observed.
RESULTS: In comparison with the normal gastric mucosa, the significantly increased expression of somatostatin mRNA positive cells was displayed in gastric carcinoma ( t = 2.681, P < 0.01). The positive signal cells were distributed in a scattered form or aggregated as a mass or a cord, and the positive cells were more significantly enhanced in poorly differentiated carcinomas than those in well and moderately differentiated carcinomas (t = 2.962, P < 0.01). The somatostatin mRNA hybridization signals in stages III and IV of gastric carcinoma were significantly higher than those in stages I and II. The results of somatostatin immunoreactivity were consistent with those of in situhybridization.
CONCLUSION: The alteration of the expression of somatostatin mRNA was associated with the deve-lopment of gastric carcinoma and may play an important role in the process of tumor differentiation.
- Citation: Zhang QX, Dou YL, Shi XY, Ding Y. Expression of somatostatin mRNA in various differentiated types of gastric carcinoma. World J Gastroenterol 1998; 4(1): 48-51
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/48.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.48
The study of the effect of endocrine hormone on the tumor development and cellular localization of the hormone DNA, and mRNA at molecular level using in situ hybridization techniques has been considered as the target of the tumor endocrinal investigation. It has been known that somatostatin involves the cell division and differentiation. However, there has been no report on somatostatin mRNA expression and its effect on normal and gastric carcinoma tissues with in situ hybridization at molecular level. In this study, in situ hybridization with digoxigenin-labelled antisense RNA probe and immunohistochemical techniques were used to investigate the alteration of somatostatin gene transcription and the expression of the above-mentioned tissues in the normal and the various differentiated types of gastric carcinoma tissues.
Fifteen normal gastric mucosa and 32 gastric carcinoma samples were obtained from the surgical operations in Henan Tumor Hospital. Before operation no patients had been treated with anti-tumor drugs. After surgery, the specimens were rinsed immediately in saline and fixed in freshly prepared 4% polyformaldehyde (PFA) for 1-4 h at 4 °C. Washed with 0.01 mol/L PBS, the specimens were immersed in autoclaved 30% sucrose overnight at 4 °C. The frozen sections and paraffin-embedded sections were prepared and used for in situ hybridization and immunohistochemistry respectively. The frozen sections were incubated for 12-16 h at 43 °C, and then preserved at -20 °C.
Washed with water, the slides were immersed in clean solution overnight. Washed with water and distilled water, the slides were roasted for 2 h at 180 °C. Coated with DEPC created gelatin, the slides were dried overnight at 37 °C.
The gastric carcinoma specimens were classified into poorly, moderately and well differentiated types, and four clinical stages (stages I-IV) according to the standard of the clinical classification for gastric carcinoma set up by the National Gastric Carcinoma Association.
The frozen sections of normal and gastric carcinoma tissues were treated with 0.1 mol/L PBS for 5-10 min, 0.1 mol/L glycine/PBS for 5 min, 0.3% Triton X-100/PBS for 15 min. Digested with 1 mg/ L proteinase K (England) in 0.1 mol/L Tris-HCl, pH 8.0, 50 mmol/L EDTA buffer for 20 min at 37 °C, the specimens were postfixed with 4% PFA for 5 min. Washed with 0.1 mol/L PBS, the specimens were immersed in freshly prepared 0.25% acetic anhydride in 0.1 mol/L triethanolamine for 10 min to reduce the background. Washed with 2 × SSC for 10 min, each slide was covered with 10 μL-30 μL hybridization solution (prehybridization procedure was omitted) containing 5% formamide, and 10% dextransulfate (Fluka), Digoxigenin-labelled SOM antisense RNA probe, 10 mmol/L Tris-HCl (pH 8.0), 0.03 mol/L NaCl, 1 mmol/L EDTA, 250 mg/L salmon sperm DNA, 10 mmol/L DTT, 0.25% PVP, 0.35% BSA, 0.24% Ficoll 400. The sections were covered with 20 mm × 20 mm Parafilm (Greenwich CT), and hybridization was performed at 43 °C for 16-20 h in a moisture chamber. After hybridization, the specimens were washed with 2 × SSC for 30 min. Following the removal of parafilm, the specimens were digested with 20 mg/L RNase A for 30 min at 37 °C to eliminate the unhybridized probe. Washed 3 times with 0.05 mol/L PBS for 5 min, the specimens were incubated with Anti-Dig-AP (Boehriger Mannheim) 1:1000 diluted in 1% BSA, 0.4% Triton X-100/0.05 mol/L PBS for 12-16 h at 4 °C. Washed 4 times with 0.01 mol/L PBS for 10 min, the specimens were stained with freshly prepared 400 mg/L NBT/20 mg/L BCIP for 3-16 h. The reaction was stopped by washing with 20 mmol/L EDTA for 30 min.
The sections were pretreated with RNase A (0.05 g/L) for 30 min at 37 °C. All procedures were the same as above except for no probe in hybridizationsolution.
Immunohistochemistry was used according to the PAP method. The samples were incubated with normal goat serum 1:10 diluted with 0.3% Triton X-100 for 30 min. Then, the somatostatin antibody (DAKO) (diluted 1:100 in PBS) was added and incubated for 24 h at 37 °C. Goat anti-rabbit IgG (diluted 1:25 in PBS) was added and incubated for 1 h at 37 °C. Then, PAP complex (diluted 1:100 in PBS) was added to the sections and incubated for 1 h at 37 °C. After that, the samples were colored in freshly prepared 0.5 g/L DAB for 10-15 min at room temperature.
Normal rabbit serum was substituted for goat anti-rabbit IgG and PBS was substituted for PAP complex.
The data were statistically analysed with t test.
Distinct hybridization signal was detected with digoxigenin-labelled antisense RNA probe in both the normal tissues and in the gastric carcinoma tissues. The hybridization signal was blue-violet, and located in cytoplasm; and there was no signal in the nucleus. The background of the hybridization specimen was colorless.
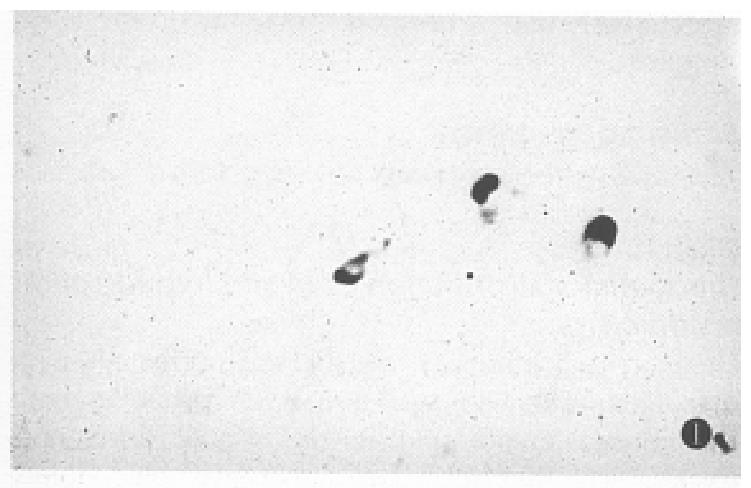
The positive signal cells were distributed in a scattered form. The hybridization signal could be seen in 13 of the 15 normal gastric mucosa specimens. However, the hybridization positive cells were less (Table 1), mainly located in the middle and the lower portion of the mucosa, and appeared in round or irregular shape (Figure 1), and some cells had a slender process.
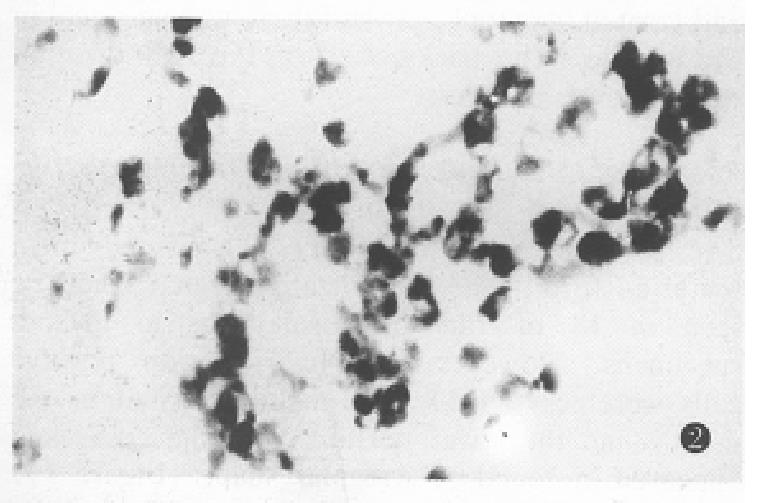
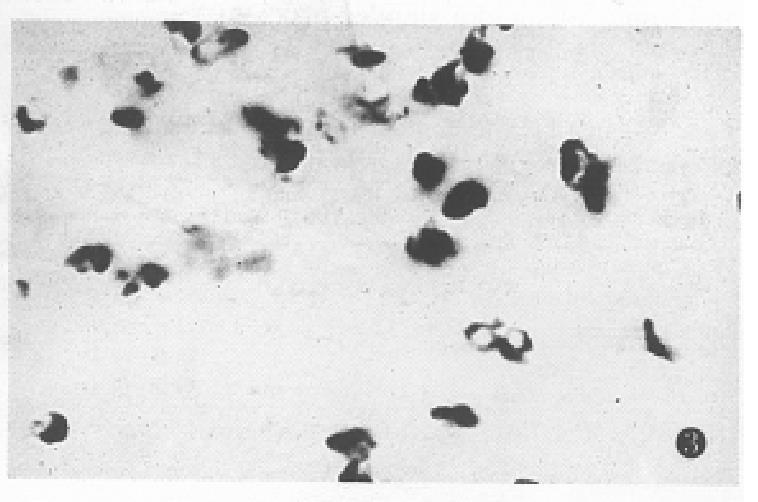
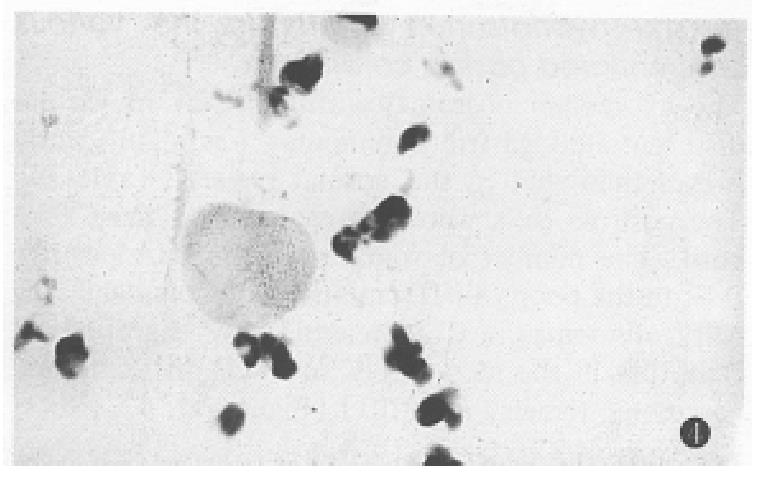
The expression of somatostatin mRNA in various differentiated gastric carcinomas was significantly higher than that in the normal tissues (Table 1). The positive cells were aggregated as a mass or a cord. The number of soma tostatin mRNA positive cells in the poorly differentiated carcinoma and ring cell carcinomas was increased more significantly than that in the moderately and well differentiated carcinoma tissues (P < 0.01) (Figure 2, Figure 3, Figure 4).
The in situ hybridization signals detected with somatostatin antisense RNA probe in various clinical stages of gastric carcinoma tissues are displayed in Table 2.
The somatostatin positive immunoreactivity appeared as brownish colored granules and was located in the cytoplasm. The positive cells in the poorly differentiated carcinoma were significantly higher than those in the moderately and the well differentiated carcinomas. The results of somatostatin immunoreactivity was consistent with those by in situ hybridization (Table 3).
| Normal gastric mucosa | Gastric carcinoma | |||
| Poorly differentiated | Moderately differentiated | Well differentiated | ||
| r | 0.874 | 0.725 | 0.836 | 0.836 |
| P | < 0.001 | < 0.001 | < 0.01 | < 0.01 |
Both in situ hybridization and immunohistochemistry were negative.
Up to date, there have been many reports about the relationship between gastroenteric hormones and endocrinal tumors in the digestive tract[1,2]. However, there have been few reports about the research of endocrinal regulation of gastroenteric non-endocrinal tumor and its mechanism with in situ hybridization. Chen[3] and Ooi[4] found endocrine granules or hormones in the common gastroenteric carcinoma cells and in the tumor endocrine cells of the digestive tract with ultrastructural and immunohistochemical techniques. However, immunohistochemical techniques could only show the gastroenteric hormone preserved in a certain kind of tumor cells, but could neither reveal whether the hormone came from protein synthesis by itself or was the exogenous substance, non distinguish if amino acid composition was similar, such as α or β cGRP[5] by proteins or polypeptides. In situ hybridization was not only the unique effective method to identify the result of immunohistochemistry, but also an index to watch the hormone synthesis process, since the alteration of mRNA transcription rate was much faster than that of the translation production[6]. In this study, the in situ hybridization with digoxigenin-labelled somatostatin antisense RNA probe was first used to study the human normal and gastric carcinoma tissues. The results displayed intense signals with clear background and no nonspecific reaction, which indicated the satisfactory results of cellular localization at molecular level for somatostatin mRNA hybridization signal positive cells in normal gastric mucosa and the various differentiated gastric carcinoma tissues.
In the previous studies, it was believed that somatostatin inhibited cell growth[7]. However,the recent studiesdemonstrated that somatostatin could stimulate tumor cell growth[8,9]. Although the exact mechanism is still unclear, at least two possible sorts of explanation can be considered. The first is the inhibition of trophic action of gastrin on mucosal cells caused by exogenous and endogenous gastrin, and the second is the effect of somatostatin on cell proliferation. Our study on various clinical stages of gastric carcinoma showed that the somatostatin mRNA hybridization signals in stages III and IV were significantly higher than those in stages IV and II. Because there was a high incidence of migration to lymphonodes and peripheral organs in stage III and IV, it was indicated that the over expression of somatostatin was significantly correlated with the development and prognosis of gastric carcinoma.
Generally, the poorer the tumor cell differentiation, the worse the prognosis. In this study, the hybridization positive cells were more significantly enhanced in poorly differentiated gastric carcinoma than those in well and moderately differentiated gastric carcinomas (P < 0.01). The results indicated that, to some extent, somatostatin mRNA and somatostatin were associated with the differentiation induction of gastric carcinoma at the level of transcription and translation. The abnormal regulation at transcription level may stop the cell differentiation at a certain stage and the differentiated features of carcinoma cells occur. It is worth considering that the alteration of the expression of somatostatin mRNA may play an important role in the process of tumor differentiation.
Project supported by the National Natural Science Foundation of China, No. 39170440 and Natural Science Foundation of Henan Province.
| 1. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [PubMed] |
| 2. | Walker FM, Lehy T, Bernuau DG, Sobhani I, Bayle D, Feldmann G, Lewin MJ. Detection of gastrin mRNA in human antral mucosa and digestive endocrine tumors by in situ hybridization: a correlative study with immunocytochemistry and electron microscopy. J Histochem Cytochem. 1992;40:1363-1372. [PubMed] |
| 3. | Chen BF, Yin H. Neuro-endocrine type of gastric carcinoma. Immunohistochemical and electron microscopic studies of 100 cases. Chin Med J (Engl). 1990;103:561-564. [PubMed] |
| 4. | Ooi A, Mai M, Ogino T, Ueda H, Kitamura T, Takahashi Y, Kawahara E, Nakanishi I. Endocrine differentiation of gastric adenocarcinoma. The prevalence as evaluated by immunoreactive chromogranin A and its biologic significance. Cancer. 1988;62:1096-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Noguchi K, Senba E, Morita Y, Sato M, Tohyama M. Alpha-CGRP and beta-CGRP mRNAs are differentially regulated in the rat spinal cord and dorsal root ganglion. Brain Res Mol Brain Res. 1990;7:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Kiyama H, Emson PC, Tohyama M. Recent progress in the use of the technique of non-radioactive in situ hybridization histochemistry: new tools for molecular neurobiology. Neurosci Res. 1990;9:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Kraenzlin ME, Ch'ng JL, Wood SM, Carr DH, Bloom SR. Long-term treatment of a VIPoma with somatostatin analogue resulting in remission of symptoms and possible shrinkage of metastases. Gastroenterology. 1985;88:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 120] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Koper JW, Markstein R, Kohler C, Kwekkeboom DJ, Avezaat CJ, Lamberts SW, Reubi JC. Somatostatin inhibits the activity of adenylate cyclase in cultured human meningioma cells and stimulates their growth. J Clin Endocrinol Metab. 1992;74:543-547. [PubMed] |
| 9. | Moyer MP, Armstrong A, Aust JB, Levine BA, Sirinek KR. Effects of gastrin, glutamine, and somatostatin on the in vitro growth of normal and malignant human gastric mucosal cells. Arch Surg. 1986;121:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |