Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.19
Revised: September 19, 1997
Accepted: October 26, 1997
Published online: February 15, 1998
AIM: To undergo apoptosis during negative and positive selection processes in rat mucosal immune system which are implicated in the pathogenesis of various mucosal diseases.
METHODS: Female Sprague-Dawley rats were given protein synthesis inhibitor, cycloheximide, intravenously or intraperitoneally, an apoptosis was recognized by morphological hallmark under light and electronmicroscopy, and the expression of proliferating cell nuclear antigen was visualized immunohistochemically.
RESULTS: The apoptosis of mucosal lymphocytes in the digestive tract, as well as in trachea, uterus and lacrimal gland was induced by cycloheximide ( > 1.0 mg·kg-1 body weight), which were located mainly in lamina propria and germinal centers of lymphoid nodules. At the same time, a portion of crypt epithelial cells of proliferating zone in small and large intestine, and the epithelial cells in genital tract were also found to undergo apoptosis. Immunostainings showed that apoptotic cells expressed proliferating cell nuclear antigen.
CONCLUSION: Apoptosis of lymphocytes in mucosal immune system can be induced by cycloheximide. This model will facilitate the understanding of normal mucosal immune system and its role in the pathogenesis of related diseases such as inflammatory bowel diseases.
- Citation: Chen XQ, Zhang WD, Song YG, Zhou DY. Induction of apoptosis of lymphocytes in rat mucosal immune system. World J Gastroenterol 1998; 4(1): 19-23
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/19.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.19
Mucosal immune system represents the most important humoral immune system of the body quantitatively[1,2]. In adults, about 80% of immunoglobulin producing immunocytes are located in the intestinal lamina propria showing the dominant role of the gut as an activated B-cell organ[1-3]. Antigenic priming of organized gut-associated lymphoid tissues, mainly includes the Peyer’s patches, and may give rise to specific secretory immunity in not only the gut, but also the respiratory tract, lacrimal, salivary and lactating mammary glands[2]. The main function of the mucosal immune system is to protect the host against pathogenic bacteria and viruses to which the mucosal surfaces are exposed. The mucosal immunocytes play an important role in the pathogenesis of inflammatory bowel diseases(IBD), gluten enteropathy and Sjögren’ s syndrome, although the mechanisms have not been elucidated yet[4,5].
Apoptosis (programmed cell death) is a process genetically controlled leading to cell suicide[6]. Peripheral T and B lymphocytes, as well as their precursors in the central lymphoid organs, may undergo apoptosis during the negative and positive selection processes. Abnormality of apoptotic machinery within the cell results in the development of various types of diseases. Resistance to apoptosis may be coupled to autoimmune diseases, and leukemia. In contrast, elevated apoptotic decay of lymphocyte has been shown to be involved in lymphopenia caused by human immunodeficiency virus infection[7]. Therefore, it is reasonable to expect that induction of apoptosis in immune system will facilitate better understanding of immune system and its role in related diseases.
In this experiment, we used a protein synthesis inhibitor cycloheximide ( CHX ) to establish a method for rapid induction of apoptosis of immuocytes in rat mucosal immune system. We focused our research on digestive tract as well as on trachea, uterus and lacrimal glands.
Cycloheximide was purchased from Serva. The monoclonal antibody against proliferating cell nuclear antigen ( PCNA ) used for immunostaining were from ZYMED Lab, Inc. VECTASTAIN ABC kits were products of VECTOR. 3’-diaminobenzidine hydrochloride (DAB) and pepsin were purchased from Sigma. All other chemicals were of regent grade.
Female Sprague-Dawley rats (PLA Animal Center of South China, Guangzhou) weighing 200 ± 20 g were maintained on a standard laboratory diet and given water ad libitum. Twenty-four hours before experiment, they were deprived of food, but accessible to water freely. After anaesthetized with ether, the rats were injected with CHX (dissolved in 0.9% sodium chloride) intravenously or intraperitoneally at dosage of 0.5, 1.0, 2.0, 3.0, 4.0, 5.0 and 10 mg·kg-1 body weight. Controls were given an equal amount of saline. Rats were sacrificed at various time points after treatment (1,2,3 and 4 h).
The tissues collected from lacrimal gland, uterus, trachea, and digestive tract (including stomach, duodenum, jejunum and ileum and colon, small portions (0.5 cm × 0.5 cm) were fixed immediately in 4% buffered-paraformaldehyde (pH 7.2) and embedded in paraffin. These were cut at 4 μm thickness and stained with hematoxylin and eosin (H & E). Apoptotic cells were identified morphologically[6,8] for shrinkage of the cells, chromatin condensation, formation of apoptotic bodies and absence of inflammation. Apoptosis incidence could be calculated randomly by counting the apoptotic bodies in lamina propria of uterus[8]. At least 200 cells per sec were counted, and three to four rats per group were used.
After the rats were sacrificed, the specimens were immediately fixed in 2.5% glutaraldehyde buffered in 0.1 M- PBS (pH 7.2) at 4 °C for at least 2 h. Tissues were washed with the same buffer and postfixed in 1% osmium tetroxide in phosphate buffer at 4 °C for 2 h, then dehydrated in graded acetone and embedded in Epon resin. After stained with uranyl acetate and lead citrate, the ultrathin sections were examined under the electronmicroscope (JEOL, JEM, 1200-EX, Japan).
Paraffin sections were deparaffined, rehydrated and digested with 0.001% pepsin in 0.01N hydrochloric acid at 37 °C for 10 min, and rinsed in 0.01 M PBS (pH 7.2) for another 10 min. The tissues were then incubated in chronological order with a primary anti-PCNA antibody ( diluted 1:100 ) at 4 °C overnight, a biotinylated-secondary antibody at 37 °C for 30 min, and avidin-biotin complex for development at 37 °C for 45 min. During incubation, the sections were washed for 10 min in PBS. Staining was developed in DAB-hydrogen dioxide and sections were counterstained with hematoxylin. Primary antibody was omitted in negative controls.
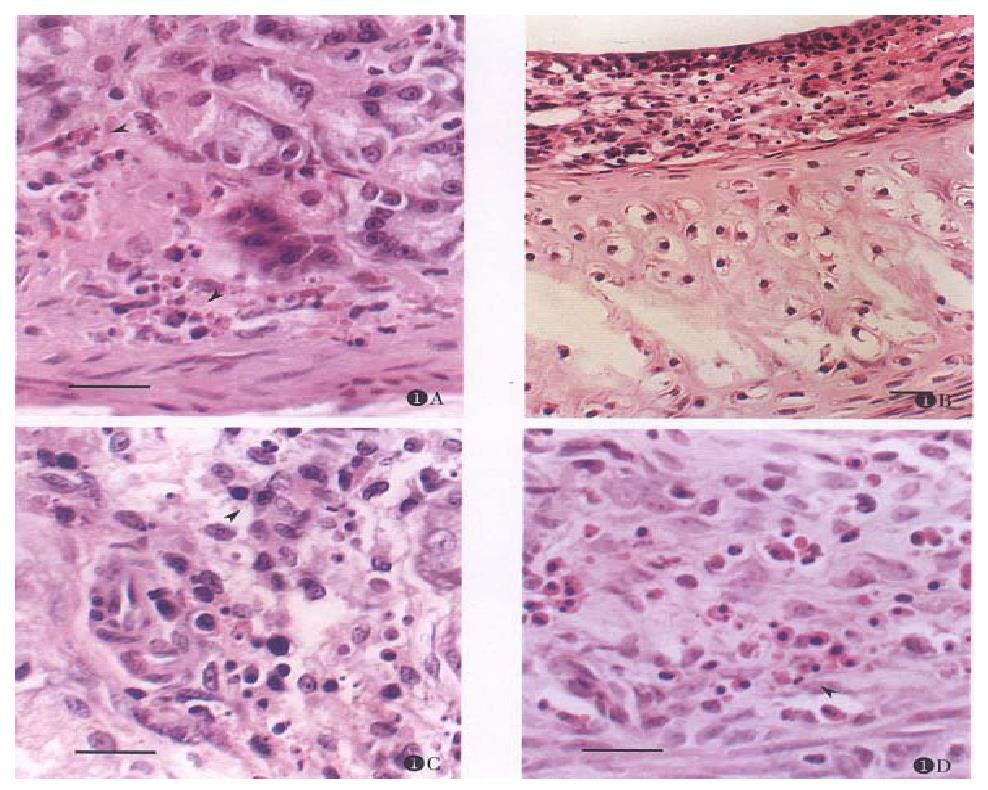
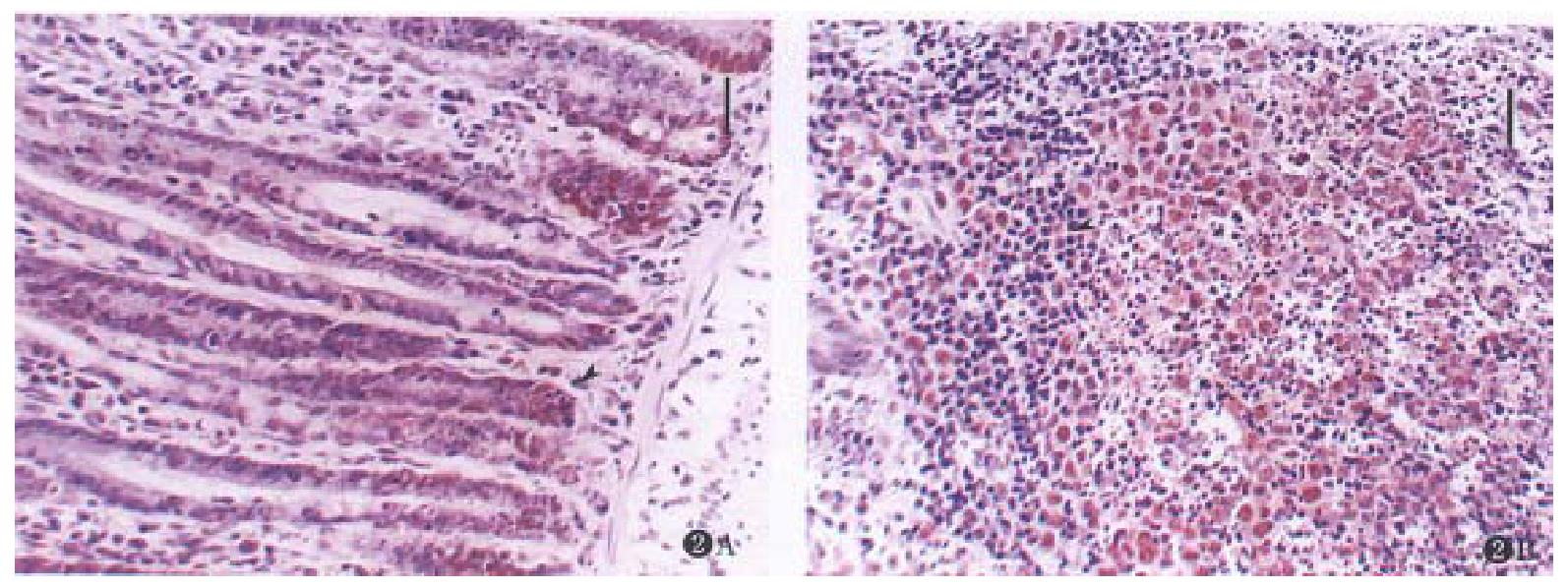
Apoptotic cells were rarely found in normal mucosal of digestive and respiratory tracts, lacrimal gland and reproductive system, even in the germinal centers of lymphoid nodules. After the rats were given CHX, apoptosis was found in the mucosa of these organs with characteristic features, and the apoptotic tissues were devoid of inflammatory response at any time point (Figure 1A, B, C, D, Figure 2A, B, Figure 3B). The induced lymphocyte apoptosis was scattered in lamina propria and clustered in lymphoid nodules. At the same time, the apoptosis of intraepithelial lymphocytes (IEL), particularly in digestive tract, was also observed. Besides, a portion of the mucosal epithelial cells were found dead, including crypt epithelial cells in the small and large intestines (Figure 2A) and epithelial cells of uterus. However, the epithelial cells lacrimal glands and trachea were still normal ( Figure 1B, C). The apoptosis of epithelium in the digestive tract was within proliferating zone of crypt epithelium (Figure 2A, B). Despite these changes, the epithelium remained in integrity, and no erosion or ulceration were seen macroscopically. Microscopically, vascular changes were found in lamina propria with capillary dilatation and the vessels packed with erythrocytes, and no thromb was observed.
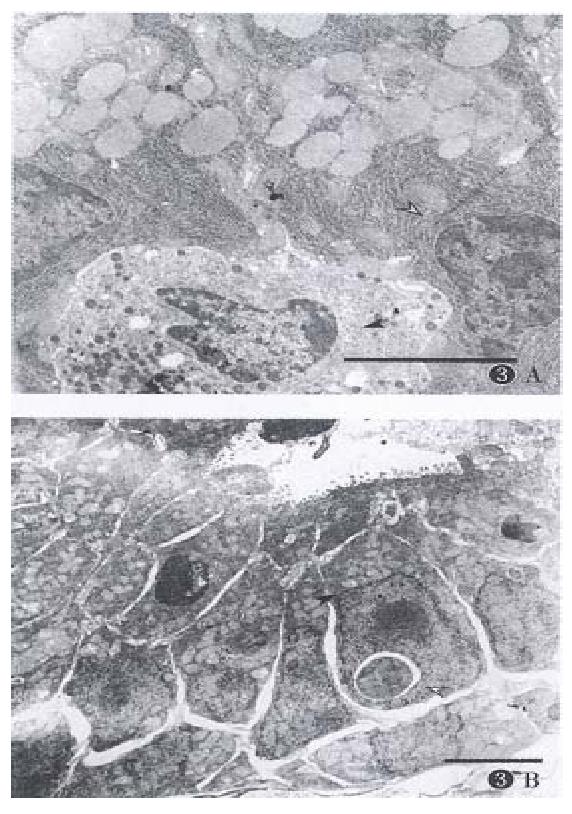
Apoptosis was defined as condensation and margination of chromatin, contraction of cytoplasm, and cell shrinkage, and the mitochondria could be seen[6,8]. In this study, at the early apoptotic stage, the mitochondria was well preserved, and its number increased as well (Figure 3B). Activated macrophages phagocytosed apoptotic bodies (Figure 1B, C). Some apoptotic bodies of lymphocytes and epithelial cells were phagocytosed by the neighboring epithelial cells.
PCNA is an important index proliferating cells[9]. The immunostaining showed strong positive expression of PCNA in lymphocytes of germinal centers, crypt epithelial cells of proliferating zone of small and large intestines (Figure 2A, B), and epithelial cells of reproductive system. The scattered lymphocyte in lamina propria in lymphoid nodules, especially in the gut were also positively expressed (Figure 3B). Fragmented nuclei were still found expressed with PCNA. Therefore, the PCNA expression was in consistent with the induced apoptosis. However, some of the lymphocytes and epithelial cells with positive PCNA expression appeared normal morphologically (Figure 3A, B). On the other hand, the cells with negative PCNA expression failed in apoptosis (Figure 3A, B), even with large dosage of CHX( > 10.0 mg·kg-1).
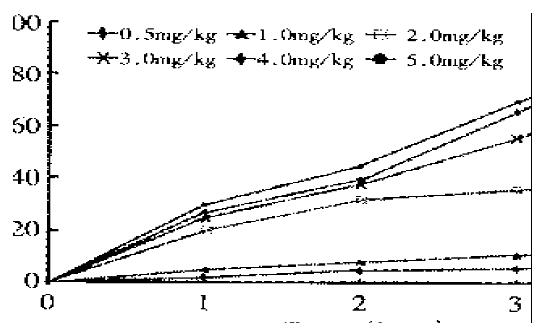
To estimate the effects of CHX on the induced apoptosis, apoptosis incidence could be calculated by counting apoptotic bodies and apoptotic cells of lamina propria in uterus. The incidence of apoptosis was dose-dependent (Figure 4). CHX 1.5 mg·kg-1 was nonlethal, and only inhibited the protein synthesis by 98% to 90% 1-3 h after treatment[8]. Initial intraperitoneal injection of CHX at a dosage of 1.0 mg·kg-1 showed insignificant effects on apoptosis induction. Apoptosis was achieved significantly in 2 h and reached its maximum 4 h after a relatively large dosage of > 2.0 mg·kg-1 body weight. In this study, the mortality rate of the rats was 33.3% (2/6) with 5.0 mg·kg-1, and up to 83.3%(5/6) with 10.0 mg·kg-1 18 h after CHX. CHX, when given intravenously or intraperitoneally, had the same effects on incidence of apoptosis.
In this study, mucosal lymphocytes in the immune system and a small portion of mucosal epithelial cells of the digestive and genital tracts could be induced to apoptosis by protein synthesis inhibitor CHX in rats. The apoptosis identified by morphologic features under light and electron-microscopy, could exhibit obviously in 2 to 4 h after the rats were treated with relatively high dosage ( > 1.0 mg·kg-1) of CHX, no cell necrosis or inflammatory changes were observed.
Lymphocytes primed by antigens in intestinal mucosal lymphoid follicles may transit through lymphatics and mesenteric lymph nodes, enter into circulation, and subsequently “homing” back to the intestinal lamina propria and other mucosal sites of respiratory tract, the female genital tract[1,2]. Peyer’s patches are inductive sites and lamina propria and IEL are effector sites. These lymphoid tissues contain more immunocytes than any other tissues of the body[1,2]. Peyer’s patches can be divided into three distinct regions: the specialized dome epithelium, the B-cell zone, and the parafollicular T-cell zone. In our experiment, most of lymphocytes in lamina propria and in the germinal centers of Peyer’s patches underwent apoptosis,together with induction of IEL apoptosis in the digestive tract. Only a few lymphocytes in the parafollicular T cell zone of Peyer’s patches were triggered to death by apoptosis. PCNA was expressed in the apoptotic cells in Peyer’s patches and lamina propria PCNA. It has been demonstrated that elevated PCNA appear in the nucleus during late G1 phase immediately before the onset of DNA synthesis, and peaked during S phase[9]. These indicate that most of the apoptotic cells induced by CHX in common immune system were activated B and T lymphocytes (at least IEL in gut mucosa)[1,2,10,11].
Epithelial cells are constantly exfoliating to lumen and replaced by cells migrating from the proli-ferating zone, and homeostasis of this process is thought to be controlled by apoptosis[6]. In our study, CHX induced crypt epithelial cells of proli-ferating zone in the intestines and in the genital tract to apoptosis. These apoptotic cells were positively expressed with PCNA. Recent studies showed that passage through S phase is required for apoptosis, and S phase is the critical period to determine cell fate either to replicate or to die[12]. It seems likely that inhibition of protein synthesis accelerates the process of cell death by inhibition of de novo micromolecular synthesis[13,14]. Alternatively, inhibition of “protective protein” synthesis of cells initiated apoptosis. Paradoxically, a number of epithelial cells at proliferating stage, examined by light microscopy, were not triggered to apoptosis by CHX in this experiment, which is consistent with the aforementioned results of lymphocytes. There might be three explanations: one is that these “normal cells” appear “normal” at the early stage of apoptosis, the second one is that the propensity of these cells to undergo apoptosis are cell-cycle-phase directed, suggesting that CHX may only interfere with a certain phase of proliferating cell cycle, and the third explanation is that the phenotype of apoptosis is not critically dependent on protein synthesis and the endonuclease involved in DNA fragmentation is already in place[15].
Although lymphocytes and epithelial cells underwent apoptosis after rats were given CHX, the monocytes/macrophages in common immune system were not affected. It has been observed that increased macrophages actively moved to and appeared in tissues where apoptotic cells and apoptotic bodies were formed in clusters. They phagocytosed the apoptotic cells and apoptotic bodies. This phenomenon renders the apoptosis distinctly different from necrosis and easily recognizable in tissue slides. In addition, before the DNA fragmentation and formation of apoptotic bodies, electronmicroscopy showed that the morphologic changes of the cells in apoptosis would take place in chronological order separating from their neighbors, increasing number of mitochondria, condensed nucleus and cytoplasm. It suggested that the increased mitochondria within cytoplasm can be an early hallmark of apoptosis. Cytoplasm of apoptotic cells was strongly stained with eosin (Figure 1B). It indicates that proliferating mitochrodria may be protective/ compensatory to apoptosis in consideration of some “protective protein” like bcl-2 protooncogen protein synthesized in mitochondria[16].
It is reported that CHX can induce apoptosis in hepatic cells[8], intestinal crypt cell of proliferating cell types in vivo[17]. The former represents the first in vivo evidence of apoptosis induced by CHX in a quiescent organ. However, our findings indicated that CHX mainly affected activated lymphocytes and epithelial cells of proliferating phase, and we failed to observe that quiescent epithelial cells and endocrine cells succumb to apoptosis by CHX. Therefore, susceptibility to apoptosis is dependent on cell types. In case of stomach the parietal cell, chief cell and ECL cell were morphologically normal (Figure 1A, Figure 3A), and some parietal cells were found in mitosis phase. Proliferating cells were frequently encountered in the isthmus region of stomach[18]. Although these negative findings were observed in our study, it is conceivable these proli-ferating cells may undergo apoptosis induced by CHX.
In summary, CHX injection can severely da-mage the body immune system of rats, especially the mucosal immune system, but it influences the mucosal epithelial and endocrine cells only slightly though injury of epithelial cells may damage the integrity of epithelium at the early stage (unpublished data). In fact injection of CHX (2.0 mg·kg-1) could cause diarrhea in rats for 3 days possibly due to enterobacterial infection, or it might be correlated with sIgA deficiency and malabsorption[19]. We also used this model to study mucosal lymphocytes involved in the mechanisms of gastric acid production ( unpublished data ). Therefore, this model will be useful in investigating the role of mucosal immune system in the pathogenesis of related diseases such as IBD, celiac diseases, and Sjögren’s syndrome, as well as turnover mechanism of mucosal immunocytes.
Project supported by Ph. D. grant from First Military Medical University, Guangzhou.
| 1. | Brandtzaeg P, Nilssen DE, Rognum TO, Thrane PS. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am. 1991;20:397-439. [PubMed] |
| 2. | Brandtzaeg P, Halstensen TS, Kett K, Krajci P, Kvale D, Rognum TO, Scott H, Sollid LM. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97:1562-1584. [PubMed] |
| 3. | van der Heijden PJ, Stok W, Bianchi AT. Contribution of immunoglobulin-secreting cells in the murine small intestine to the total 'background' immunoglobulin production. Immunology. 1987;62:551-555. [PubMed] |
| 4. | Schreiber S, Raedler A, Stenson WF, MacDermott RP. The role of the mucosal immune system in inflammatory bowel disease. Gastroenterol Clin North Am. 1992;21:451-502. [PubMed] |
| 5. | Hansen BU, Ericsson UB, Henricsson V, Larsson A, Manthorpe R, Warfvinge G. Autoimmune thyroiditis and primary Sjögren's syndrome: clinical and laboratory evidence of the coexistence of the two diseases. Clin Exp Rheumatol. 1991;9:137-141. [PubMed] |
| 6. | Gerschenson LE, Rotello RJ. Apoptosis: a different type of cell death. FASEB J. 1992;6:2450-2455. [PubMed] |
| 7. | Kroemer G, Martínez C. Pharmacological inhibition of programmed lymphocyte death. Immunol Today. 1994;15:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Chen XQ, Zhang YL, Song YG, Tan XH, Zhang WD, Zhou DY. A method for rapid induction of apoptosis in rat spleen by cycloheximide. Chin J Cell Biol. 1996;18:144-145. |
| 9. | Celis JE, Celis A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: subdivision of S phase. Proc Natl Acad Sci USA. 1985;82:3262-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 441] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Guy-Grand D, Griscelli C, Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974;4:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 258] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Terui Y, Furukawa Y, Kikuchi J, Saito M. Apoptosis during HL-60 cell differentiation is closely related to a G0/G1 cell cycle arrest. J Cell Physiol. 1995;164:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Martin SJ, Lennon SV, Bonham AM, Cotter TG. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990;145:1859-1867. [PubMed] |
| 14. | Evans DL, Dive C. Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes. Cancer Res. 1993;53:2133-2139. [PubMed] |
| 15. | Borner MM, Myers CE, Sartor O, Sei Y, Toko T, Trepel JB, Schneider E. Drug-induced apoptosis is not necessarily dependent on macromolecular synthesis or proliferation in the p53-negative human prostate cancer cell line PC-3. Cancer Res. 1995;55:2122-2128. [PubMed] |
| 16. | Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2650] [Cited by in RCA: 2713] [Article Influence: 77.5] [Reference Citation Analysis (1)] |
| 17. | Ijiri K, Potten CS. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987;55:113-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 284] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Crabbé PA, Heremans JF. Selective IgA deficiency with steatorrhea. A new syndrome. Am J Med. 1967;42:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 2.2] [Reference Citation Analysis (0)] |