Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.14
Revised: September 25, 1997
Accepted: October 14, 1997
Published online: February 15, 1998
AIMS: To isolate mouse CCR5 cDNA (muCCR5) and study its expression in vivo.
METHODS: Marathon PCR was used to isolate muCCR5 cDNA and two animal models were designed to investigate the gene expression in vivo, one was mouse fulminant hepatitis induced by Propionibacterium acnes (P.acnes) and the other was that with delayed type hypersensitivity reaction (DTH). A specific GST-NH2-terminus of muCCR5 fusion protein antibody F(ab’)2 was prepared and clarified. RT-PCR and immunohistochemical analysis were used to observe the expression level of CCR5 gene in mice.
RESULTS: A positive reaction of mouse macrophage was found in DTH but not expressed in P.acnes induced fulminant hepatitis by RT-PCR and immunohistochemical analysis.
CONCLUSION: This muCCR5 expression may be involved in an allergic processmediated by cellular immunity but not acute inflammatory reaction induced by P.acnes.
- Citation: Guo BY, Zhang SY, Mukaida N, Harada A, Kuno K, Wang JB, Sun SH, Matshshima K. CCR5 gene expression in fulminant hepatitis and DTH in mice. World J Gastroenterol 1998; 4(1): 14-18
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/14.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.14
Leukocytotaxis plays an important role in immune system surveillance and chronic inflammation. Locally produced chemotactic factors are thought to be critical in this directed migration. The chemotactic family can be divided into two subfamilies: (1) the 1st subfamily having the first two of the conserved cystein residues separated by another amino acid, the CXC (α) chemokines, and the second with CC unseparated the CC (β) chemokine. The β-chemokine receptors are some G-protein coupled receptors with seven transmembrane domains and share a high degree of amino acid homology in their putative transmembrane domains. Recent researches indicated that the entry of HIV into target cells required the participation of at least two cell surface molecules: one was CD4+ which was utilized by all HIV strains as the primary virus receptor through a high affinity interaction with the viral envelope protein. However, the CD4+ alone was not sufficient for virus entry, and some additional cell surface molecules, termed cofactor, for example, CCR5, CCR3, CCR2b and fusin[1-5] were found to mediate the entry of HIV-1 into the host cells. CCR5 can express in monocytes, macrophages, and primitive T cells, and bind to β-chemokine RANTES, MIP-1α and MIP-1β. Expression of CCR5 in conjunction with CD4+ in a variety of cell types renders them permissive for infection through M-tropic envelope proteins. Meanwhile, CCR5 and CD4+ are expressed in several cells to mediate the M-tropic HIV strain envelope to form syncytia[6]. The M-tropic HIV-1 strain is most sensitive to changes in the first extracellular loop, and therefore, to understand the CCR5 expression is very important. Unfortunately, we found that under the normal condition, mouse CCR5 gene expressed only in a few cell lines and at a very low level in vivo. In this study, we used the model of DTH (delayed type hypersensitivity) and a fulminant hepatitis induced by P. acnes for observing the CCR5 expression in vivo and analyzed the mechanism of the gene expression.
Human embryonic kidney cell line 293 was cultured in DMEM ( Nissui Pharmaceutical Co, LTD, ) medium containing 10% fetal calf serum and 50 U/mL penicillin G and 50 μg/mL streptomycin.
Mouse peritoneal macrophages isolated 3 days after pentose injection, and their total RNA was prepared using RNA 201 B (Cinna/Biotec, Houston, TX). Macrophage total RNA was reverse-transcribed by RT-PCR using a random primer (Takara Shuzo Co. LTD ) in the presence of RNase inhibitor (Promega). Corresponding to the sequences of the highly conserved region between the second and fifth transmembrane domains within human MCP-1 and mouse MIP-1α receptors, the sense primer was (PTM2) 5’-GCGAATTCTGGCCAT(CT)TCTGA(CT)CTGCT(CT)TT(CT)CT-3’, and the antisense primer was (PTM5) 5’GCAAGCTT(GC)A(CT)(GT)GG(AG)TTGA(CT)(AG)CAGCAGTG(AC)GT-3’. 5’-RACE and 3’-RACE reactions were performed to isolate the full-length mouse CCR5 cDNA by means of Marathon cDNA amplification kit ( Clontech, CA). In brief, the first PCR reaction was carried out using primer “R1” and the primer adapter 1. The second PCR reaction was performed with the internal primer “R2” of CCR5 and the other primer adaptor 2. The specific primers of mouse CCR5 were as follows: (R1) 5’-GGATCAGGCTCAAGATGACC-3’, (F1) 5’-ACACTCAGTATCATTTCTGG-3’. PCR products were digested with appropriate restriction enzymes and subcloned into pBluescript SKII+ (Stratagene). DNA sequencing reaction was performed by a PCR procedure employing fluorescent dideoxynucleotides and analyzed by a model 373A automated sequencer (Applied Biosystem).
For construction of the expression vector of mouse CCR5, the coding region of mouse CCR5 gene was amplified by PCR with specific primers and cloned into pcDNA3 (Invitrogen Corporation). The 5’ primer for PCR was designed to generate Kozak sequence, and the constructs were introduced into a human embryonic kidney cell line 293 by the calcium phosphate coprecipitation method modified by Chen[7]. Transfected cells were selected in the presence of a neomycin analogue, G-418 (Life Technologies, Inc), at a concentration of 500 ng/L in complete medium.
For preparing a recombinant GST protein fused with NH2-terminal portion of muCCR5 cDNA, the NH2-terminal extracellular binding domain encoding Metl-Leu38 from the ORF region of muCCR5 cDNA was obtained by polymerase chain reaction, and then cloned into EcoRI and BamHI restriction sites of the GST-fusion protein expression vector pGEX-IN. The recombinant DNA was transferred into E. coli HB 101 competent cells (Toyobo competent). The expression and purification of a GST fusion protein were induced with 0.1 M IPTG (isopropylthio-β-galactoside, Wako Pure Chemical Industries, LtD) for 5 h, put on glutathione-sepharose 4B affinity column (Pharmacia Biotech AB Upsala, Sweden), and then eluted with 5 mM of reduced glutathione.
Two New Zealand white rabbits were immunized with 100 μg of the GST-NH2-terminal of muCCR5 fusion protein in CFA (Iatron, Tokyo, Japan); first time, the footpads were injected, and other 9 times at biweekly intervals were given s.c.. One week after the final immunization, rabbits were bled, sera were obtained, and fractionated into IgG by a protein A agarose column (Pharmacia-Biotech, Upsala, Sweden). A portion of the IgG fraction was further digested with pepsin (Sigma Chemical, St. Louis, MO) and the F(ab’)2 fragment was obtained by sequential chromatographies using a protein A affinity and a sepharose 12gel filtration column as previously described[8].
BALB/c female mice of 6-7 weeks were used. The hair of abdomen were shaved, 5% Picryl Chloride 100 μL was painted on the surface of abdomen, after 5 days 1% picryl chloride was painted on the right ear, 24 and 48 h later the effect of DTH was evaluated, and then the tissues were taken for preparing total RNA with RNA 201. RT-PCR was run according to Normua[9] (94 °C for 1 min, 55 °C for 1 min, and 72 °C for 1.5 min, with a total of 30 cycles).
Mice were injected with 1 mg of heat-killed P.acnes into the tail vein. Seven days later the indicated dosages of LPS were administered intravenously[10]. At 3 h from LPS challenge, three to four mice were killed to obtain the liver. Liver tissues were fixed in 4% paraformaldehyde for 2 h before being transferred to 70% ethanol and subsequent paraffin embedding.
For immunohistochemical analysis, paraffin-embedded tissues were dewaxed with histo-clear (National Diagnostics, Tokyo, Japan) and dehydrated through graded concentrations of ethanol. After being treated with trypsin and blocked with 1% skim milk, the tissue sections were covered with 40 ng/L of anit-muCCR5 antibody F(ab’)2 and GST over night at 4 °C, incubated with biotinylated swine anti-rabbit IgG, and then reacted with ALP substrate solution (Vector Laboratories, Burllingame, CA) containing 1 mM levamisole and solution I, II, III for 40 min at room temperature. Finally, the sections were rinsed and counterstained with 1% methyl green.
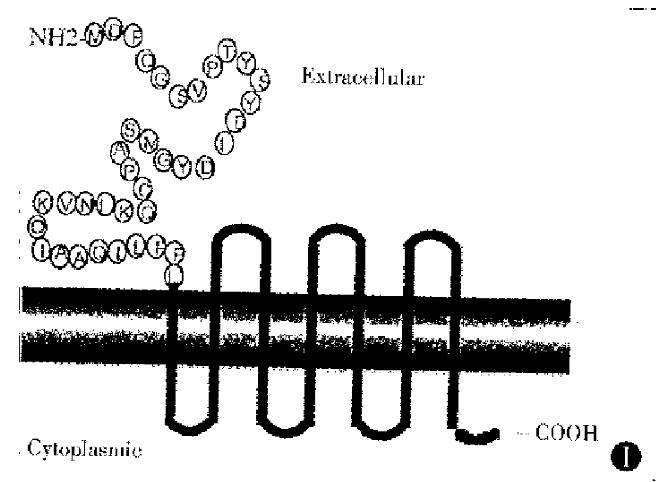
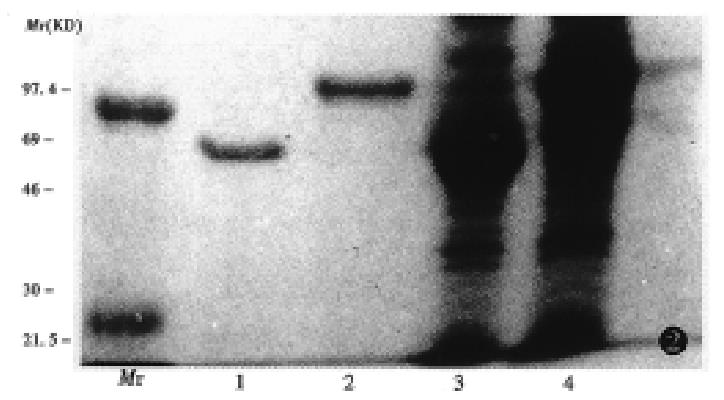
A full length cDNA sequence and amino acid sequence of muCCR5 was cloned by Marathon PCR from the poly (A)+RNA of mouse peritoneal macrophages. The ORF region of muCCR5 consisted of 355 amino acid, and had a 82% homology with human CCR5. A GST fusion protein of the NH2-terminal 38 amino acids of the first loop of extracellular part of muCCR5 was expressed as cytoplasmic protein in E. coli (Figure 1). A corresponding fusion protein of 30 kD and GST molecule of 26 kD were observed on 15% SDS-PAGE with Coomassie blue-staining (Figure 2). In general, 3 mg-5 mg GST-NH2-terminus of muCCR5 fusion protein was produced from 200 mL E. coli solution.
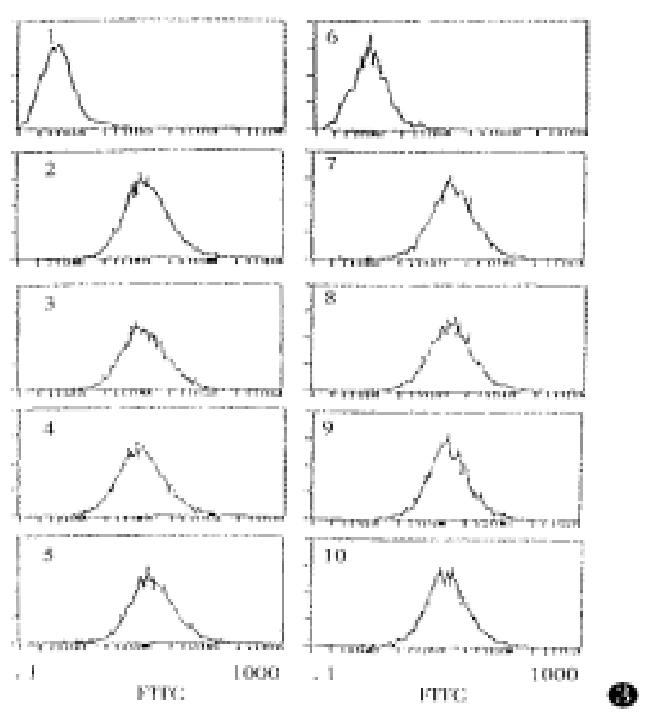
An experiment was performed to examine the cross reaction of the anti-muCCR5 F(ab’)2 with other receptors by means of the muCCR5 293 transfectant, and the results showed that the anti-muCCR5 F(ab’)2 could bind with GST-NH2-muCCR5 fusion protein but not human CCR1, CCR2, CCR3, CCR4, Fusin and mouse IL-8 (Figure 3), suggesting the specificity of the anti-muCCR5 F(ab’)2 to muCCR5.
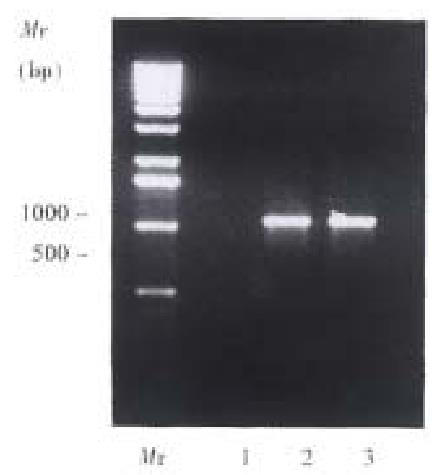
The expression of muCCR5 mRNA in DTH was confirmed by RT-PCR, with the sense primer 5’-ATGGATTTTCAAGGGTCAGTTC-3’ and antisense primer 5’-TCATAAACCAGTAGAAACTTC-3’. The results showed that after induction with 1% picryl chloride for 24 and 48 h, muCCR5 could express in spleen cells and the negative result was found in fulminant hepatitis induced by P.acnes (Figure 4).
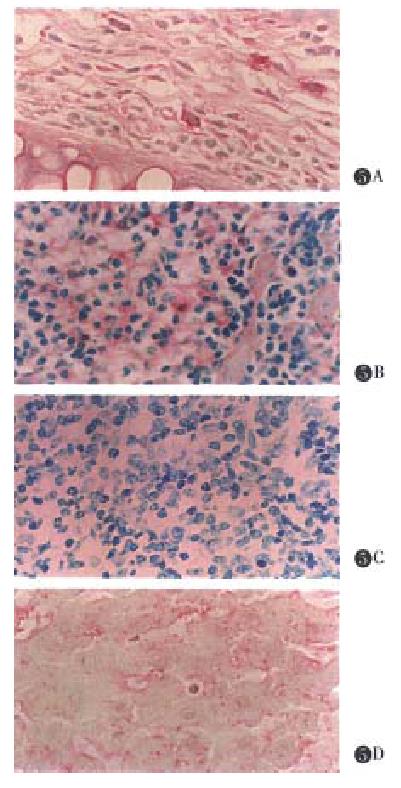
In the normal mice, CCR5 expression level was very low or nil, and muCCR5 was not expressed in fulminant hepatitis induced by P.acnes reaction ( unpublished data ). The Immunohistochemical analysis revealed that muCCR5 clearly expressed on ear at the point of picryl chloride induction (Figure 5A). A weakly positive result was found in spleen macrophages in DTH reaction induced by picryl chloride (Figure 5B), but a negative result of DTH reaction induced by picryl chloride was found in normal experiment (Figure 5C) and a liver Kupffer cells also showed a positive result in DTH reaction (Figure 5D).
In this study we first cloned mouse CCR5 full length cDNA sequence from peritoneal macrophages ploy (A) +RNA with Marathon PCR, then constructed the fusion protein of GST with 5’ terminal extracellular binding domain 38 amino acid of muCCR5 and successfully have it expressed in E. coli. In the Northern analysis, we could not find expression of muCCR5 in vivo. In order to understand the expression of CDR5 in vivo, two animal models were designed, one was mouse fulminant hepatitis induced by P.acnes and the other was that with delayed type hypersensitivity reaction ( DTH ). In previous studies, P.acnes induced transient increase in serum TNF-a levels but not those of IL-1ra, IL-1 and IL-6. However subsequent LPS challenge induced the elevated serum levels of all these cytokines and the peak serum IL-1ra level was 20 times that of serum IL-1 levels. Immunohistochemical analysis demonstrated that IL-1ra was predominantly produced by hepatocytes during the priming phase by P.acnes and eliciting phase by LPS challenge[10]. The responsiveness of alveolar lymphocytes to recombinant IL-2 was evaluated by 3H-thymidine uptake in the presence and absence of P.acnes. P. acnes stimulates IL-2 production and IL-2 receptor induction in alveolar lymphocytes from patients with active sarcoidosis[11]. In our study of the expression of muCCR5 in P.acnes induction, we did not observe a positive reaction, which indicated that muCCR5 gene expression did not involve an acute inflammatory process. In the DTH reaction, the results showed that muCCR5 had expressed in mouse macrophages. No matter some factors might induce a DTH condition, in this case, cell immunity mediated CCR5 gene expression and HIV-1 in infected macrophages more easily. This is a interesting problem which needs further studies.
There have been some reports about the DTH reaction, for example, RANTES produced by IFN-α and TNF-α induction has already been described in a number of studies and clearly IFN-α and TNF-α have a synergic action. INF-d stimulated macrophage to express RANTES and TNF-α stimulated cells to express RANTES gene. It is well known that RANTES is a ligand of CCR5. In the DTH state, macrophages not only express ligand but also express its receptor. In this case, if the ligand binds to its receptor, what physiological changes will happen? Or the ligand (RANTES) thus produced may induce receptor expression; this is another interesting problem. Th-2 cytokines such as IL-4 and IL-10, have been demonstrated to inhibit DTH granule formation and increased production of these cytokines was associated with impaired DTH reaction[11-15]. Th-2 cytokines may affect CCR5 expression of macrophages, because a high concentration of Th-2 cytokines can alter the recruitment of immune cells to DTH granules at least partly by inhibiting CCR5 expression.
| 1. | Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2825] [Cited by in RCA: 2794] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 2. | Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2487] [Cited by in RCA: 2434] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 3. | Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2173] [Cited by in RCA: 2062] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 4. | Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2244] [Cited by in RCA: 2176] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 5. | Paxton WA, Martin SR, Tse D, O'Brien TR, Skurnick J, VanDevanter NL, Padian N, Braun JF, Kotler DP, Wolinsky SM. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 451] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath PD, Wu L, Mackay CR, LaRosa G, Newman W. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 1836] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 7. | Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3464] [Cited by in RCA: 3318] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 8. | Morohashi H, Miyawaki T, Nomura H, Kuno K, Murakami S, Matsushima K, Mukaida N. Expression of both types of human interleukin-8 receptors on mature neutrophils, monocytes, and natural killer cells. J Leukoc Biol. 1995;57:180-187. [PubMed] |
| 9. | Nomura H, Nielsen BW, Matsushima K. Molecular cloning of cDNAs encoding a LD78 receptor and putative leukocyte chemotactic peptide receptors. Int Immunol. 1993;5:1239-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:3043-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 151] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Devergne O, Marfaing-Koka A, Schall TJ, Leger-Ravet MB, Sadick M, Peuchmaur M, Crevon MC, Kim KJ, Schall TT, Kim T. Production of the RANTES chemokine in delayed-type hypersensitivity reactions: involvement of macrophages and endothelial cells. J Exp Med. 1994;179:1689-1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 161] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Tsicopoulos A, Hamid Q, Varney V, Ying S, Moqbel R, Durham SR, Kay AB. Preferential messenger RNA expression of Th1-type cells (IFN-gamma+, IL-2+) in classical delayed-type (tuberculin) hypersensitivity reactions in human skin. J Immunol. 1992;148:2058-2061. [PubMed] |
| 13. | Devergne O, Emilie D, Peuchmaur M, Crevon MC, D'Agay MF, Galanaud P. Production of cytokines in sarcoid lymph nodes: preferential expression of interleukin-1 beta and interferon-gamma genes. Hum Pathol. 1992;23:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1072] [Cited by in RCA: 1148] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 15. | Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171:115-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 514] [Article Influence: 14.7] [Reference Citation Analysis (0)] |