Published online Feb 15, 1998. doi: 10.3748/wjg.v4.i1.10
Revised: November 22, 1997
Accepted: December 16, 1997
Published online: February 15, 1998
AIM: To study VEGF mRNA expression in gastric carcinoma and to clarify the association of its expression with the clinicopathologic features of the disease.
METHODS: In situ hybridization (ISH) and histochemistry were used to examine and analyze the expression of VEGF mRNA and antigen, and microvessel count (MVC) in 28 cases of gastric carcinomatous tissue in combination with clinical materials.
RESULTS: Ninteen of 28 gastric carcinomas were positive for VEGF mRNA. VEGF mRNA was mainly expressed in malignant cells and not in normal epithelium of gastric mucosa. Its expression was further increased in tumor cells adjacent to tumor necrosis zones, where stromal cells expressed VEGF mRNA occasionally. There was a close correlation between MVC and VEGF mRNA positivity (P < 0.005). High VEGF mRNA levels were significantly associated with serosal invasion, lymph node metastasis and TNM staging ( P < 0.05, respectively).
CONCLUSION: VEGF mRNA expression is associated with tumor invasion and metastasis by stimulating angiogenesis in gastric carcinoma.
- Citation: Tao HQ, Lin YZ, Wang RN. Significance of vascular endothelial growth factor messenger RNA expression in gastric cancer. World J Gastroenterol 1998; 4(1): 10-13
- URL: https://www.wjgnet.com/1007-9327/full/v4/i1/10.htm
- DOI: https://dx.doi.org/10.3748/wjg.v4.i1.10
Solid tumors are composed of two distinct but interdependent compartments, the malignant cells themselves and the vascular and connective tissue stroma that they induce and in which they are dispersed. Stroma provides the vascular supply that tumors require for obtaining nutrients, gas exchange, and waste disposal. Thus, any increase in tumor mass, either primary or metastatic, must be accompanied by stroma formation[1].
The mechanism by which tumors induce stroma has caused considerable attention in recent years. Most work have focused on one aspect of stroma generation, angiogenesis, and have called attention to a variety of tumor-secreted “angiogenesis factor”, particularly VEGF, that is likely to play an important role in both angiogenesis and other aspects of tumor stroma generation. VEGF is an Mr 34000-42000 KD, disulfide-linked glycoprotein synthesized by several human and animal cell types, both normal and neoplastic[2-4]. VEGF was originally recognized for its ability to increase the permeability of the microvasculature to circulating macromolecules. More recently, VPF has also been shown to be a selective endothelial cell mitogen, and therefore has been alternatively called VEGF[5-7]. By alternating splicing of mRNA, four different molecular species with 121, 165, 189 and 206 amino acids were determined.
VEGF was originally discovered as a tumor-secreted protein, and its role in tumor development were investigated[5,8]. It is considered to play an important role in tumor biology in at least two ways: as a vascular permeability factor and/or endothelial growth factor. As a potent permeability factor, VEGF promotes extravasation of plasma fibrinogen, leading to the formation of a fibrin network which serves as a substratum for cell migration during angiogenesis. While as an endothelial growth factor, VEGF stimulates endothelial cell proliferation and is likely to induce the formation of new blood vessels[9].
VEGF is synthesized and secreted by a variety of tumor cells in tissue culture and by several transplanted animal tumors in vivo[7,10]. Elevated expression of VEGF in human tumors have been reported in most tumors. However, there has been few reports on the VEGF expression in gastric carcinoma.
In this study, we examined the VEGF expression in gastric carcinoma by means of in situ hybridization ( ISH ) and compared it with the pathological features of gastric carcinoma in order to determine whether VEGF is overexpressed in gastric carcinoma compared with the normal stomach tissues and its role in the invasion and metastasis of gastric carcinoma.
Twenty-eight patients with gastric carcinoma who underwent surgical resections at Ruijin Hospital were studied, including 19 men and 9 women, aged from 38 to 78 (mean age 56.2 ± 11.9) years. None had received chemotherapy or radiotherapy before surgical resection. A tumor sample and a normal part of the stomach were obtained during surgical resection. The samples were each divided into two pieces. One was subjected to fixation in 10% formalin for histological examination and immunohistological test, and the other was used for ISH, i.e., tissues were fixed for 4 h in 4% paraformaldehyde in phosphate-buffered saline (PBS), pH 7.4, at 4 °C and were then transferred to 30% sucrose in PBS, pH 7.4, overnight at 4 °C. The tissues were then frozen in OCT (Miles Inc.) and stored at -70 °C until use for VPF/VEGF mRNA expression analysis. Pathological features of the patients were obtained from the pathological reports and clinical records. Histological analysis of the tumor was done by experienced pathologists without knowing the analytical results. The differentiation grading was based on the predominant findings.
The pBluescript II SK + construct with the VEGF insert (a gifted from Dr. Yamauich, Japan) was linearized with Bam H I and transcribed in vitro from the T7 polymerase promoter to yield the antisense RNA probe. The same construct was linearized with Eco R I and transcribed from the T3 polymerase promoter to yield the sense (control) probe. The transcription reaction was performed in the presence of 0.35 mM digoxigenin-UTP ( Boehringer Mannheim GmbH, Biochemica, Mannheim, Germany) to yield digoxigenin-labeled RNA probe.
In situ hybridization was performed using a digoxigenin nucleic acid detection kit with some modifications. Frozen sections were cut at 10 μm, fixed for 20 min in 4% paraformaldehyde/0.1M PBS, and rinsed in PBS. Specimens were then treated with 0.1% proteinase K, fixed in 4% paraformaldehyde/0.1M PBS again, acetylated in 0.1M triethanolamine and 0.25% acetic anhydride, and dehydrated through a graded ethanol series. Sections were prehybridized for 2 h at 53 °C with 50 μL of prehybridization fluid containing 50% formamide, 0.3 M NaCl, 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 10% dextran sulfate, 1 × Denhardts solution ( 0.02% Ficoll, 0.02% BSA, 0.02% polyvinyl pyrrolidone), and 0.025% salmon sperm DNA in a humidified chamber. After the prehybridization, hybridization was performed in buffer containing prehybridization fluid and 1.0 mg·L-1 of either antisense or sense probe at 53 °C for 16 h. Next, sections were washed at 37 °C for 30 min in 4 × SSC (150 mM NaCl and 15 mM sodium citrate) and 30 min in 2 × SSC/0.05%SDS, digested with RNase (20 mg·L-1) at 37 °C for 15 min, and washed again at 37 °C for 30 min in 2 × SSC/0.05% SDS, and washed at 37 °C for 30 min in 1 × SSC, 0.5 × SSC, respectively. Then, blocking was performed in blocking buffer containing 1% BSA for 30 min. After the blocking buffer was removed, the solution containing 1:1000 sheep anti-digoxigenin Fab fragment conjugated to alkaline phosphatase and 1% BSA was placed on each section. Sections were placed in a humidified chamber and incubated at room temperature for 1 h. After the sections were rinsed, a coloring reaction was performed using 225 μg·mL-1 nitroblue tetrazolium salt and 175 mg·L-1 5-bromo-4-chloro-3-indolyl-phosphate. When the color reaction was appropriate, stop it by washing in buffer solution. The section was washed, dehydrated and mounted.
The methods of microvessel staining and counting were as described previously[11].
The clinicopathological features between VEGF expression and nonexpression groups were analyzed by Fisher’s test. The correlation between MVC and VEGF expression was evaluated with Student’ s t test.
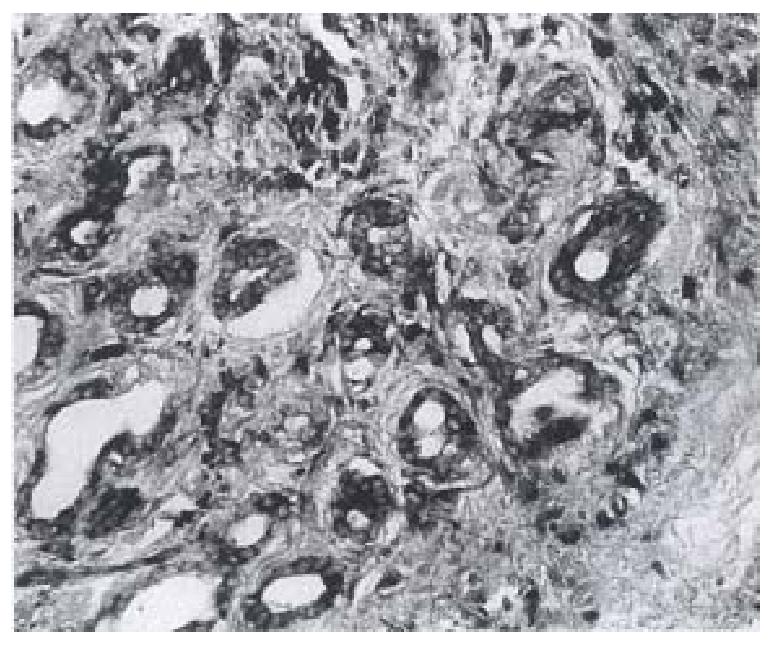
Twenty-eight cases of human gastric carcinoma were studied by ISH with both antisense and sense (control) probes to VEGF. VEGF mRNA was expressed in the malignant epithelium of 19 of the 28 patients (19/29, 67.9%). Generally, VEGF mRNA was distributed homogene ously and intensely throughout the tumor. VEGF was expressed by tumor cells under microscopy (Figure 1). However, it is noteworthy that in the periphery of necrotic foci of the gastric cancer, labeling with the VEGF antisense probe was distinctly intensified in tumor epithelium adjacent to the necrotic foci. The tumor stroma cells also labeled for VEGF mRNA. Stromal labeling was distinctly focal as compared to the labeling regularly observed over tumor cells and usually occur red in stroma immediately adjacent to the foci of overt tissue necrosis. Labeled stromal cells included fibroblasts and smooth muscle cells. However, vascular endothelium did not express detectable VEGF mRNA. No specific cellular labeling was seen with the VEGF antisense in normal epithelium of gastric mucosa from these cases, nor did tumors label with the VEGF sense probe.
VEGF mRNA expression in gastric carcinoma was compared with the clinical and histological features of the tumors. The comparison between VEGF mRNA expression groups and nonexpression groups is shown in Table 1.
| Characteristics | VEGF mRNA(+) | VEGF mRNA(-) | P |
| Vascularity | |||
| MVC | 18.1 ± 7.8 | 12 ± 5.9 | < 0.005 |
| Serosal invasion | |||
| Positive | 16 | 3 | < 0.005 |
| Negative | 3 | 6 | |
| Lymph node metastasis | |||
| Positive | 17 | 4 | < 0.05 |
| Negative | 2 | 5 | |
| Histologic type | |||
| Differentiated | 2 | 2 | < 0.05 |
| Undifferentiated | 17 | 7 | |
| Other metastasis | |||
| Positive | 3 | 0 | < 0.05 |
| Negative | 16 | 9 | |
| TNM staging | |||
| I | 1 | 4 | |
| II | 9 | 3 | < 0.05 |
| III | 6 | 2 | |
| IV | 3 | 0 |
VEGF mRNA expression was significantly correlated with serosal invasion and lymph node metastasis, although no correlation was found between its expression and histologic type, and other metastasis. Incidences of VEGF mRNA expression were increased as the pathologic stage progressed.
Factor VIII was stained in tumor vascular endothelial cell. Microvessel counts varied from 3 to 38 counts/ × 200 field (average, 15.6 counts). Table 1 shows the correlation between the MVC and VEGF mRNA status. The MVC in VEGF mRNA positive tumors was significantly higher than that in VEGF nonexpression tumors.
VEGF antigen was mainly identified in the cytoplasm of cancer cells. Expression rate of VEGF antigen was 46.4% (13/28). Although it was lower than that of VEGF mRNA, there was a significant correlation between their staining intensity ( r = 0.487, P < 0.05).
Brown et al[12] examined 2 cases of gastric adenocarcinomas on VEGF mRNA and observed a detectable signal for VEGF in all them using ISH. In this paper, 19 of the 28 primary human gastric carcinoma expressed VEGF mRNA as judged by ISH, the expression rate was 67.9%. VEGF mRNA was mainly detected in malignant glands but not in normal epithelium. This is different from the phenomena that VEGF mRNA expression was detected both in tumor and normal tissue in liver[13] and lung[14], and it is suggested that the VEGF expression seems to be a characteristic of the malignant phenotype in gastric carcinoma.
It is worth notice that there are some differences in the strongly stained areas between VEGF mRNA and antigen. Tumor cells stained strongly for VEGF antigen were observed more often in the invasion front than in the tumor center, inversely, VEGF mRNA was expressed more often in the tumor center adjacent to the zones of tumor necrosis than in the invasion front. VEGF mRNA expression elevation around the necrotic area of the tumor has been reported in several other human cancers. Rapid cell proliferation in the center of a tumor can lead to increased interstitial pressure, which may lead to compression closure of capillaries and consecutive tumor necrosis. Tumor hypoxia has been reported to increase expression of VEGF mRNA in a variety of cultured tumor cells[15]. Therefore, it is likely that, in the development of necrosis, hypoxia may increase the VEGF mRN expression.
Some studies had demonstrated the correlation between the expression of VEGF mRNA and tumor invasion and metastasis. Recently, Zhang et al[16] reported that the transfection of this growth factor gene into a tumor cell line expressing a low level of VEGF proteins altered tumor cells more angiogenesis and more progressive in the nude mouse model without change of cell growth rate in vitro. In this study, we observed a significant difference of MVC between VEGF mRNA expression group and nonexpression group. The incidences of serosal invasion and lymph node metastasis in tumor which expressed VEGF mRNA were higher than that of tumors with non-VEGF mRNA expression. The tumors with liver or ovarian metastasis are VEGF mRNA positive. Moreover, the rate of VEGF mRNA expression in the advanced tumors is higher than that in early stage tumors. All these results indicate that VEGF mRNA may play an important role in tumor angiogenesis, invasion and metastasis of gastric carcinoma.
VEGF mRNA expression was observed in 19 cases, but still was not detected in 9 cases. This suggests that angiogenesis is not simply controlled by the presence of VEGF but is mediated by several angiogenic inducers. To date, many angiogenic factors have been found, including basic fibroblast growth factor, transforming growth factor-β , and tumor necrosis factor α . In fact, there has been a lot of reports on bFGF expression in gastric carcinoma. Therefore, it is likely that vascularization in some tumors is related to other angiogenic factor. If VEGF is indeed responsible for gastric cancer angiogenesis, therapeutic strategies to inhibit its activity using either specific antibodies or antisense RNA may allow clinicians to treat not only the malignant cells within a tumor but also the vascular supply of the tumor. In conclusion, the identification of VEGF that correlates with angiogenesis in gastric cancer may provide a basis for targeting this angiogenic factor to inhibit vascularization of tumors.
| 1. | Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931-10934. [PubMed] |
| 2. | Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 667] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2734] [Cited by in RCA: 2679] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 4. | Dvorak HF, Orenstein NS, Carvalho AC, Churchill WH, Dvorak AM, Galli SJ, Feder J, Bitzer AM, Rypysc J, Giovinco P. Induction of a fibrin-gel investment: an early event in line 10 hepatocarcinoma growth mediated by tumor-secreted products. J Immunol. 1979;122:166-174. [PubMed] |
| 5. | Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1485] [Cited by in RCA: 1480] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 6. | Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3536] [Cited by in RCA: 3555] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 7. | Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 916] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 8. | Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1588] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 9. | Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 612] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 10. | Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van de Water L, Senger DR. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991;174:1275-1278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 355] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 11. | Maeda K, Chung YS, Takatsuka S, Ogawa Y, Onoda N, Sawada T, Kato Y, Nitta A, Arimoto Y, Kondo Y. Tumour angiogenesis and tumour cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer. 1995;72:319-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727-4735. [PubMed] |
| 13. | Suzuki K, Hayashi N, Miyamoto Y, Yamamoto M, Ohkawa K, Ito Y, Sasaki Y, Yamaguchi Y, Nakase H, Noda K. Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res. 1996;56:3004-3009. [PubMed] |
| 14. | Ohta Y, Endo Y, Tanaka M, Shimizu J, Oda M, Hayashi Y, Watanabe Y, Sasaki T. Significance of vascular endothelial growth factor messenger RNA expression in primary lung cancer. Clin Cancer Res. 1996;2:1411-1416. [PubMed] |
| 15. | Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3206] [Cited by in RCA: 3224] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 16. | Zhang HT, Craft P, Scott PA, Ziche M, Weich HA, Harris AL, Bicknell R. Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst. 1995;87:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 188] [Article Influence: 6.3] [Reference Citation Analysis (0)] |