Published online Mar 7, 2025. doi: 10.3748/wjg.v31.i9.99459
Revised: January 8, 2025
Accepted: January 18, 2025
Published online: March 7, 2025
Processing time: 210 Days and 3.2 Hours
In this paper, the mechanism of the Wnt/β-catenin pathway is introduced, and the process and principle of the experiment conducted by Huang et al is exp
Core Tip: We introduce the process and principle of the experiment conducted by Huang et al and discuss the reliability of the conclusion that Calculus bovis inhibits M2 tumor-associated macrophage polarization via Wnt/β-catenin pathway modulation to suppress liver cancer.
- Citation: Chen JQ, Lan X. Calculus bovis inhibits liver cancer via the Wnt/β-catenin pathway. World J Gastroenterol 2025; 31(9): 99459
- URL: https://www.wjgnet.com/1007-9327/full/v31/i9/99459.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i9.99459
Globally, liver cancer is sixth most commonly diagnosed cancer and has the fourth highest mortality rate of all cancers[1]. Generally, liver cancer can be classified into primary or secondary liver cancer. Primary liver cancer can be further divided into hepatocellular carcinoma (HCC), cholangiocarcinoma (CCA), and mixed liver cancer. HCC accounts for approximately 90% of liver cancer cases. Secondary liver cancer refers to cancer that begins in another part of the body and metastasizes through the blood or other means to the liver. Liver metastases can be caused by intestinal cancer, breast cancer, or any other type of cancer. According to previous studies, liver cancer is predicted to affect more than 1 million people worldwide by 2025. This condition is a significant public health and economic burden worldwide[2]. Despite many breakthroughs in the diagnosis and treatment of this disease, patients with liver cancer still have a poor prognosis. Thus, more efficient and cost-effective treatments are urgently needed.
Many treatments are available for liver cancer, but surgery remains the most common. Despite radical resection of liver cancer, patients with resectable liver cancer still have numerous problems, such as postoperative liver cancer metastasis, a high recurrence rate, and a poor prognosis. Advanced HCC patients who are unsuitable for transplantation or have failed local therapy are more likely to be treated with first-line drugs such as sorafenib and lenvatinib[3]. However, drug resistance limits their use. Research shows that sorafenib is effective in only 35%-43% of patients and that most patients suffer diarrhea and skin reactions on their hands and feet within 6 months[4]. Hence, finding a cancer treatment that has a curative effect and minimal side effects is an important new direction for the future of liver cancer treatment.
In 1982, Nusse and Varmus[5] discovered a new gene that was called “Wnt” while studying the oncogenic mechanisms of mouse mammary tumor viruses. Since then, interest in the Wnt/β-catenin pathway has steadily increased worldwide. According to evidence-based medicine, abnormal activation of this signaling pathway has a significant effect on cancer-related mortality. In addition, cancer onset, progression, and malignant transformation, among many other aspects, are closely correlated with aberrant Wnt/β-catenin signaling[6]. Notably, there is growing evidence that abnormal WNT/β-catenin signaling promotes the incidence of liver cancer, including HCC and CCA, the most common primary liver cancers in adults[7]. Consequently, targeting Wnt/β-catenin signaling can serve as a novel therapy for liver cancer. However, the application of Wnt/β-catenin signaling inhibitors for liver cancer treatment remains limited[8].
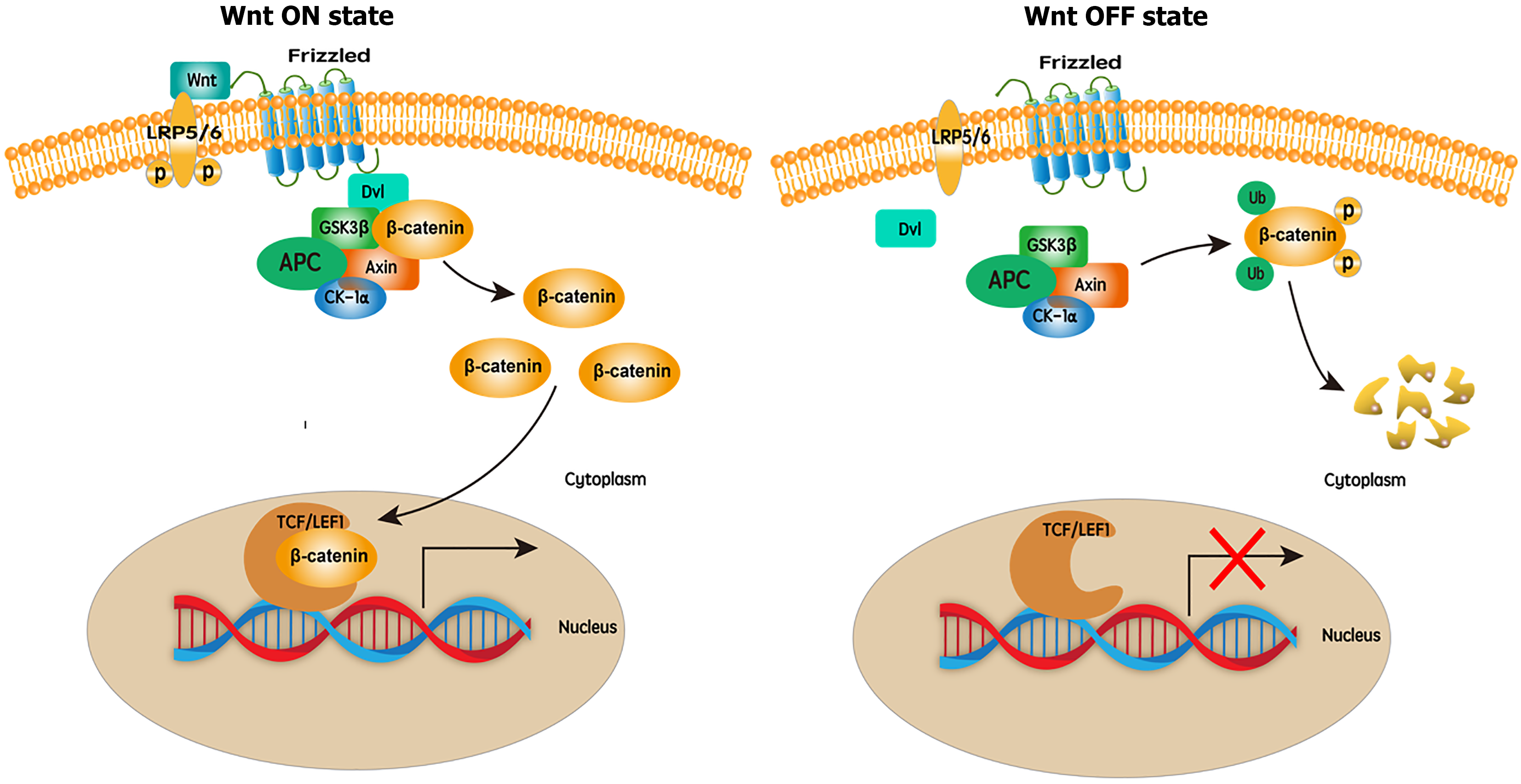
Wnt signaling is mediated by both canonical and noncanonical pathways, depending on the involvement of β-catenin. The canonical pathway is the Wnt/β-catenin pathway. The noncanonical pathway includes planar cell polarity and Ca2+ pathways, which are crucial for embryonic development, cell fate determination, proliferation, and migration[7,9]. After activation of the Wnt/β-catenin pathway, Wnt proteins bind to two molecules on the surface of cells: Frizzled (FZD) and low-density lipoprotein receptor-related proteins 5/6. Next, dishevelled binds to the cytoplasmic part of FZD, where it is phosphorylated and recruits the destruction complex, which is a tertiary complex comprising Axin, adenomatous polyposis coli, casein kinase I isoform-α, and glycogen synthase kinase-3. The destruction complex of β-catenin is subsequently disrupted, leading to the accumulation of β-catenin in the cytoplasm. β-catenin translocates into the nucleus and interacts with T-cell factor/lymphoid enhancer factor-1 (TCF/LEF1), activating the TCF/LEF1 transcription complex. Once the TCF/LEF1 transcription complex is activated, the transcription of target genes begins, including c-Myc and cyclin D1 which are closely related to the occurrence of liver cancer. When the Wnt ligand is absent, the destruction complex phosphorylates β-catenin, which is then degraded by proteasomes, resulting in deactivation of the TCF/LEF1 transcription complex[7,9]. The detailed process of the Wnt/β-catenin pathway is shown in Figure 1.
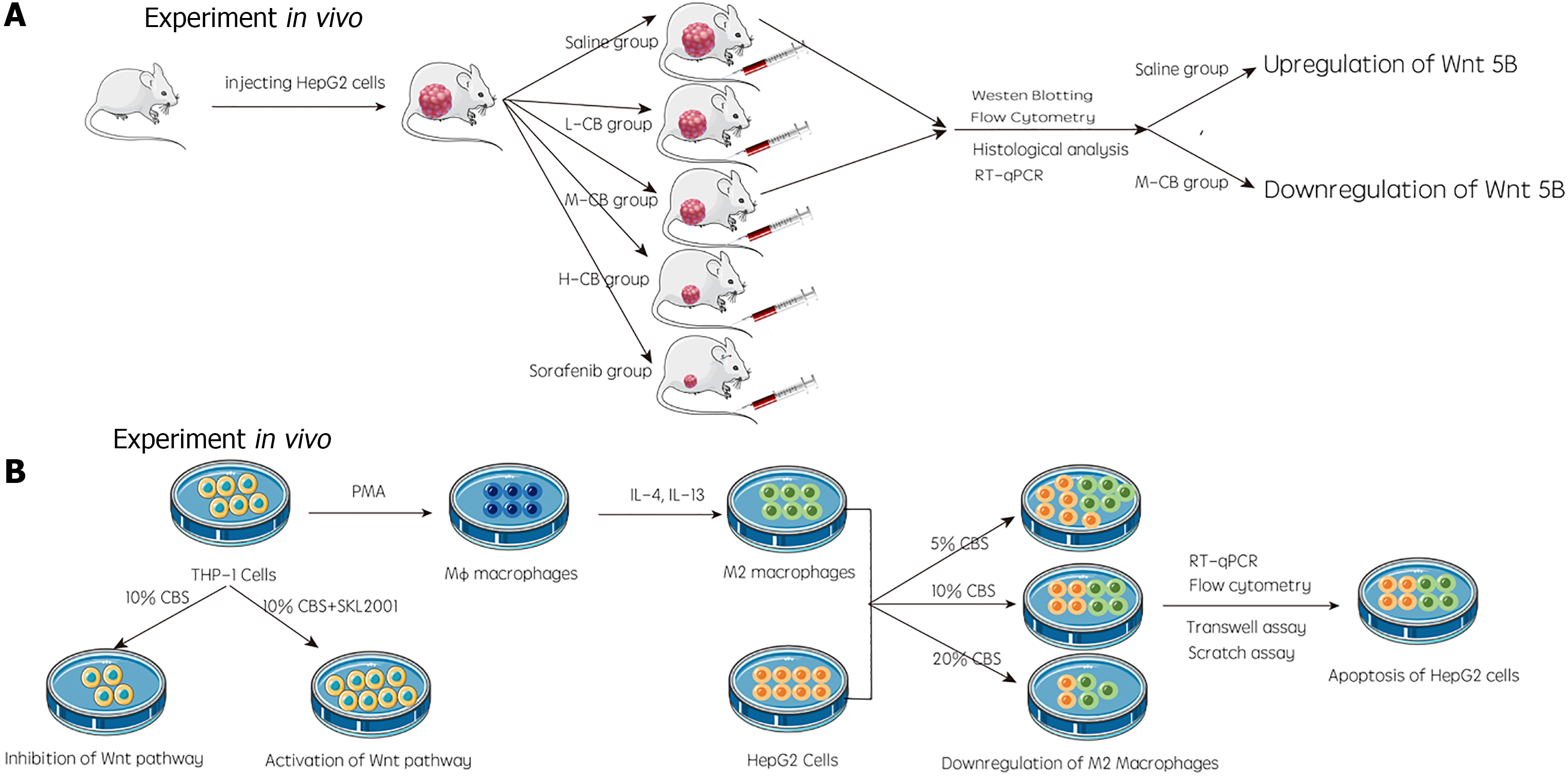
The study conducted by Huang et al[10] proposed a novel method to disrupt the Wnt/β-catenin signaling cascade. This carefully conducted study provides evidence that Calculus bovis (C. bovis) can suppress liver cancer by inhibiting M2 tumor-associated macrophage polarization via the Wnt/β-catenin pathway. An earlier study from the corresponding author of Huang et al[10] reported that C. bovis stimulated the apoptosis of SMMC-7721 cells (primary liver cancer cells) by network pharmacology and in vitro experiments[11]. To further validate this conclusion, Huang et al[10] explored the anticancer mechanism of C. bovis by network pharmacology, in vitro experiments, and in vivo experiments. In an in vivo study by Huang et al[10], a liver cancer animal model was constructed by injecting nude mice with HepG2 cells (a liver cancer cell line), and the mRNA and protein concentrations of Wnt5B, β-catenin, and Axin2 in intravenous blood sample were measured via Western blot and RT-qPCR. Simultaneously, M2 macrophage marker CD206 was examined by flow cytometry. In Huang et al’s in vitro experiments[10], induced M2 macrophages and HepG2 cells were treated with different concentrations of C. bovis-containing serum, and Wnt5B, β-catenin, and Axin2 levels were detected via Western blot and RT-qPCR. In addition, transwell and scratch assays were conducted to examine the invasion and migration capabilities of liver cancer cells, and the M2 macrophage marker CD206 was detected by flow cytometry. To further determine whether C. bovis can suppress the Wnt/β-catenin pathway, SKL2001 (a recognized activator of this pathway) was added to THP-1 cells. These results demonstrated that C. bovis could reduce M2 polarization and inhibit the Wnt/β-catenin pathway. The detailed experimental process of the study by Huang et al[10] is shown in Figure 2.
Traditional Chinese medicine has been used in China for more than 2000 years, and many Chinese herbal medicines, such as Poria and Astragali Radix, have been applied in clinical practice for HCC therapy in China[1]. In addition, several Chinese herbal medicines, including Jiedu Recipe, frankincense and myrrh, have been reported to inhibit liver cancer through Wnt/β-catenin signaling[12,13]. To our knowledge, there are no other published studies about the traditional Chinese medicine C. bovis suppressing liver cancer through the Wnt/β-catenin pathway, which indicates the originality of the study by Huang et al[10]. However, further experimental validation by other experimental teams is needed to prove the repeatability of the antihepatoma effect of C. bovis.
The results of this study[10] raise numerous questions that will stimulate further research, such as the side effects of C. bovis. Furthermore, does this approach inhibit other growth signaling pathways, such as insulin/IGF1, that cross talk with the Wnt/β-catenin cascade and appear important in hepatic cancer development[14]? Moreover, the migration, invasion, proliferation, and metastasis of tumor cells are likely regulated by other Wnt signaling pathways. Does C. bovis interfere with these processes as well? In 2021, researchers from the same laboratory as the corresponding author of this study by Huang et al[10] proposed that C. bovis can promote the apoptosis of primary liver cancer cells. This study by Huang et al[10] further explored the antihepatoma mechanism of C. bovis in vivo and the inhibition of M2 tumor-associated macrophage polarization via the Wnt/β-catenin pathway. Since C. bovis suppresses liver cancer, further clinical research can be conducted to determine whether C. bovis can be used to treat patients with this destructive disease.
| 1. | Liao X, Bu Y, Jia Q. Traditional Chinese medicine as supportive care for the management of liver cancer: Past, present, and future. Genes Dis. 2020;7:370-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 3. | Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Dika IE, Abou-Alfa GK. Treatment options after sorafenib failure in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:273-279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1227] [Cited by in RCA: 1313] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 6. | Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu C, Wang C, Ye L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 430] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 7. | He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 8. | Wang W, Smits R, Hao H, He C. Wnt/β-Catenin Signaling in Liver Cancers. Cancers (Basel). 2019;11:926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 9. | Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK, Giles FJ. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. 2017;10:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 507] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 10. | Huang Z, Meng FY, Lu LZ, Guo QQ, Lv CJ, Tan NH, Deng Z, Chen JY, Zhang ZS, Zou B, Long HP, Zhou Q, Tian S, Mei S, Tian XF. Calculus bovis inhibits M2 tumor-associated macrophage polarization via Wnt/β-catenin pathway modulation to suppress liver cancer. World J Gastroenterol. 2024;30:3511-3533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (6)] |
| 11. | Zhang Z, Zeng P, Gao W, Wu R, Deng T, Chen S, Tian X. Exploration of the Potential Mechanism of Calculus Bovis in Treatment of Primary Liver Cancer by Network Pharmacology. Comb Chem High Throughput Screen. 2021;24:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Guo BJ, Ruan Y, Wang YJ, Xiao CL, Zhong ZP, Cheng BB, Du J, Li B, Gu W, Yin ZF. Jiedu Recipe, a compound Chinese herbal medicine, inhibits cancer stemness in hepatocellular carcinoma via Wnt/β-catenin pathway under hypoxia. J Integr Med. 2023;21:474-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 13. | Lu X, Mao J, Wang Y, Huang Y, Gu M. Water extract of frankincense and myrrh inhibits liver cancer progression and epithelialmesenchymal transition through Wnt/βcatenin signaling. Mol Clin Oncol. 2023;19:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |