Published online Mar 7, 2025. doi: 10.3748/wjg.v31.i9.98027
Revised: November 24, 2024
Accepted: January 23, 2025
Published online: March 7, 2025
Processing time: 247 Days and 6.1 Hours
Metabolic-associated fatty liver disease (MAFLD) is characterized by lipid accumulation in hepatocytes and is closely associated with oxidative stress. Increasing clinical evidence indicates that MAFLD is linked to bone metabolic disorders, including osteoporosis. Recent studies indicate that the expression profiles of liver circular RNAs (circRNAs) are altered in MAFLD. However, the effects of these changes on bone metabolism remain poorly understood.
To investigate the effects and mechanism of differently expressed circRNAs secreted by the liver on osteogenic differentiation in MAFLD.
RNA sequencing was performed to identify highly expressed circRNAs in the liver, validated by quantitative real-time reverse transcription polymerase chain reaction, and localized using fluorescence in situ hybridization (FISH). A mouse model induced by a high-fat diet was used to simulate MAFLD.
CircSOD2 was significantly upregulated in liver tissues and primary hepatocytes from subjects with MAFLD. CircSOD2 was induced by oxidative stress and attenuated by antioxidants in the mouse model. In addition, circSOD2 was delivered from hepatocytes to bone marrow mesenchymal stem cells (BMSCs). Furthermore, circSOD2 inhibited the osteogenic differentiation of BMSCs and in vivo bone formation by sponging miR-29b. Moreover, miR-29b inhibition reversed the stimulatory effect of circSOD2 silencing on osteogenic differentiation of BMSCs and in vivo bone formation. Mechanistically, the interaction between circSOD2 and miR-29b confirmed through a luciferase reporter assay and the co-localization in the cytoplasm evidenced by FISH indicated that circSOD2 acted as a sponge for miR-29b.
This study provides a novel mechanism underlying the liver-bone crosstalk, demonstrating that circSOD2 upregulation in hepatocytes, induced by oxidative stress, inhibits osteogenic differentiation of BMSCs by sponging miR-29b. These findings offer a better understanding of the relationship between MAFLD and osteoporosis.
Core Tip: Emerging evidence indicates that metabolic-associated fatty liver disease (MAFLD) closely associated with oxidative stress, is linked to bone metabolic diseases. Altered expression profiles of liver circular RNAs (circRNAs) have been observed in MAFLD. This study explores the upstream and downstream mechanisms of circSOD2 upregulation in osteogenesis by identifying differentially expressed circRNAs in MAFLD livers. Results demonstrated that circSOD2 upregulation in hepatocytes induced by oxidative stress inhibits osteogenic differentiation of bone marrow mesenchymal stem cells by sponging miR-29b. These findings provide a better understanding of the relationship between MAFLD and osteoporosis from the perspective of circRNAs.
- Citation: Li LP, Chen XY, Liu HB, Zhu Y, Xie MJ, Li YJ, Luo M, Albahde M, Zhang HY, Lou JY. Oxidative stress-induced circSOD2 inhibits osteogenesis through sponging miR-29b in metabolic-associated fatty liver disease. World J Gastroenterol 2025; 31(9): 98027
- URL: https://www.wjgnet.com/1007-9327/full/v31/i9/98027.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i9.98027
Nonalcoholic fatty liver disease (NAFLD) is characterized by the accumulation of lipids in hepatocytes and is closely linked with insulin resistance, dysregulated lipid metabolism, inflammation, and oxidative stress[1]. Increasing evidence indicates that NAFLD alters the secretion of endocrine factors from the liver, including cytokines, hepatokines, and inflammatory mediators, which affects metabolic processes in extrahepatic organs and tissues[2]. Recently, experts have advocated for the term metabolic-associated fatty liver disease (MAFLD) as a more accurate descriptor of this patho
Over the past decades, MAFLD has been linked to an increased risk of cardiovascular diseases, diabetes, and bone metabolic diseases[5]. Maintaining the metabolic balance between bone formation and absorption is critical for preserving bone health and bone mass[6]. Osteogenic differentiation of mesenchymal stem cells (MSCs), an important stage of bone formation, is precisely regulated by growth factors, hormones, and cytokines[7]. Disruption of this balance, such as through impaired osteoblast differentiation, can lead to bone metabolic diseases. Osteoporosis is characterized by low bone mineral density (BMD) and deterioration of bone microarchitecture, resulting in increased bone fragility and fracture risk[8]. The relationship between MAFLD and low BMD or osteoporosis remains controversial despite growing clinical research. For instance, a large-scale cross-sectional study by Li et al[9] reported a significantly higher prevalence of osteoporotic fractures in middle-aged and older adult men with MAFLD in China. Conversely, a meta-analysis of five cross-sectional studies with significant heterogeneity found no clear association between BMD and MAFLD[10]. How
Circular RNAs (circRNAs) are a novel class of noncoding RNAs generated through back-splicing of precursor mRNAs and are characterized by covalently closed continuous loops without a terminal 5'cap and 3′polyadenylate tail[15]. circRNAs are highly stable, conserved, and tissue-specific[16]. They regulate various cellular processes, including cell proliferation and differentiation, through diverse mechanisms[17]. Recent studies have highlighted the regulatory roles of circRNAsin the osteogenic differentiation of MSCs[18] and secondary osteoporosis[19]. Additionally, there is growing evidence suggesting that circRNAs are abundantly expressed in the liver, and their altered expression is correlated with the severity of MAFLD[20,21]. To the best of our knowledge, no studies have investigated the effects of circRNA alte
Thus, we hypothesized that circRNAs secreted by hepatocytes via endocrine pathways mediate the effects of MAFLD on bone metabolism. This study aimed to investigate the impact and mechanisms of differentially expressed circRNAs in hepatocytes on the osteogenic differentiation of MSCs in the context of MAFLD, thereby elucidating the relationship between MAFLD and osteoporosis.
This study was conducted following protocols approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine. Written informed consent was obtained from all adult participants. Surgically resected liver tissues and serum samples were collected from patients undergoing hepatic hemangioma surgery at the hospital. Patients diagnosed with MAFLD were categorized as the MAFLD group (n = 5), while those with simple hepatic hemangioma were assigned to the control group (n = 5). Liver histology was independently evaluated by two blinded pathologists according to the 2018 Practice Guidance of the American Association for the Study of Liver Diseases. Other liver disease etiologies, including viral, autoimmune, cholestatic, genetic, alcoholic, and drug-induced diseases, were excluded.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Institute of Health Sciences. Healthy 8-week-old male C57BL/J6 mice were purchased from the Laboratory Animal Center of Zhejiang University. The mice were maintained under a standard 12 hours/12 hours light/dark cycle and randomly allocated into two groups. The MAFLD group was fed with high-fat diet (HFD, 60% kcal from fat, D12492, Research Diet, United States) for 24 weeks, while the control group was fed a normal diet. The investigators were blinded to the group allocation.
Total RNAs were extracted using the TRIzol reagent. After isolation and purification, approximately 5 μg of total RNA was subjected to ribosomal RNA depletion using a Ribo-Zero™ rRNA Removal Kit (Illumina Inc.) and subsequently transcribed into fluorescent cRNA using the Arraystar Super RNA Labeling Kit according to the manufacturer’s in
Primary hepatocytes and bone marrow mesenchymal stem cells (BMSCs) were cultured in RPMI1640 and Dulbecco's Modified Eagle Medium (DMEM), respectively, both supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37 °C, 5% CO2, and 95% humidity. For co-culture, BMSCs were seeded in the upper chamber, and hepatocytes were seeded in the lower chamber of a 24-well transwell plate. For osteogenic induction, BMSCs were treated with osteogenic medium (DMEM medium supplemented with 0.1 μM dexamethasone, 50 mM ascorbic acid, and 10 mM β-glycerophosphate) after cells reached 70%-80% confluence (Chemicals and reagents were listed in the Supplementary material).
The small interfering RNA targeting circSOD2 (si-circSOD2) and adenoviral circSOD2 (Ad-circSOD2) were commercially constructed by RiboBio (Guangzhou, China). Corresponding negative controls (si-NC and Ad-NC) were included. The cells were transfected with si-circSOD2 and Ad-circSOD2 in Opti-MEM™ I reduced serum medium using Lipofec
After osteogenic induction for 7 days, alkaline phosphatase (ALP) staining was performed using a commercial kit, according to the manufacturer’s instructions. For ALP activity assay, cells were lysed with 0.5% Triton X-100 for 1 hour at 4 °C. The lysates were centrifuged, and ALP activity in the supernatant was measured colorimetrically using an ALP assay kit (Jiancheng, Nanjing, China). Total protein was quantified using the BCA Protein Assay Kit (Thermo Fisher Scientific Inc.). ALP activity was normalized to the total protein and expressed as nmol/minute/mg protein.
After osteogenic induction for 14 days, BMSC mineralization was evaluated using Alizarin Red staining. The cells were fixed with 4% paraformaldehyde for 30 minutes and then stained with 40 mM alizarin red S (pH 4.0) for 15 minutes at room temperature. After rinsing with distilled water to completely remove the unbound stain, the cells were visualized and imaged using a light microscope and digital camera. After drying, staining was eluted with 10% hexadecylpyri
Liver tissues were clipped into appropriate size and fixed in 4% paraformaldehyde. After dehydration and transparency, the samples were embedded in paraffin wax and sliced into 5 μm sections. The sections were dewaxed and stained with hematoxylin & eosin (H&E). Oil Red O staining was performed using an Oil Red O dye kit (Beyotime, Shanghai, China) according to the manufacturer's protocol.
Intracellular reactive oxygen species (ROS) levels were measured using a ROS assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Hepatocytes were incubated in 2 mL of serum-free DMEM supplemented with 10 μM 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) at 37 °C for 20 minutes. After removal of the DCFH-DA-containing medium, the hepatocytes were detached with trypsin, centrifuged, and resuspended. Fluorescence levels were quantified using flow cytometry at excitation and emission wavelengths of 488 and 525 nm, respectively.
Total RNA was extracted using TRIzol reagent (Invitrogen) following the manufacturer's protocol, and RNA concentration was determined using a spectrophotometer (Nanodrop, Thermo Scientific). Complementary DNA (cDNA) was synthesized with PrimeScript RT Master Mix (Takara Bio, Otsu, Japan). qRT-PCR was performed on an ABI Prism 7500 system (Applied Biosystems, Foster City, CA, United States) using SYBR Green QPCR Master Mix (Takara Bio). The 10 μL reaction system included 2 μL cDNA and 5 μL of SYBR green (Takara Bio). Cycling conditions were as follows: 40 cycles of denaturation at 95 °C for 5 seconds and amplification at 60 °C for 24 seconds. For circRNAs, total RNAs were incubated with or without 3 U/μg of RNase R (Epicentre, San Diego, CA, United States) at 37 °C for 20 minutes. Then, RNAs were subsequently purified using the RNeasy MinElute Cleanup Kit (Qiagen). All reactions were performed in triplicate and normalized to GAPDH or U6. Relative expression level was calculated using the 2-ΔΔCtmethod (Primers were listed in the Supplementary material).
Cells were fixed with 4% paraformaldehyde for 15 minutes and permeabilized with 0.5% Triton X-100 for 5 minutes at room temperature. Fluorescence-labeled probes specific for circSOD2 were incubated with cells overnight at 37 °C. FAM-labeledcircSOD2probes and Cy3-labeled miR-29bprobes were designed and synthesized by RiboBio (Guangzhou, China). Signal detection was performed using a fluorescence in situ hybridization (FISH) Kit (RiboBio, Guangzhou, China) according to the manufacturer’s instructions. The images were acquired on a Nikon A1Si Laser Scanning Confocal Microscope (Nikon Instruments, Inc., Tokyo, Japan).
RNA immunoprecipitation (RIP) was performed using a Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, United States). HEK-293T cells were transfected with argonaute-2 (AGO2) plasmid or empty vector (HanBio, Shanghai, China) using PEI transfection reagent (Sigma-Aldrich, St. Louis, MO, United States). After 48 hours, approximately 1 × 107 cells were pelleted by centrifugation and then resuspended in 100 μL of RIP Lysis Buffer containing RNase inhibitors and protease inhibitors. The cell lysates were incubated with rabbit anti-IgG or anti-AGO2 (Millipore, Boston, MA, United States) antibodies overnight at 4 °C. The lysates were then treated with proteinase K buffer, and the immunoprecipitated RNA was extracted using the RNeasy MinElute Cleanup kit (Qiagen), followed by reverse transcription using the PrimeScript RT Master Mix (TaKaRa, Tokyo, Japan). CircSOD2 Levels were detected by RT-qPCR.
RAP experiments were performed using an RNA antisense purification (RAP) technology kit (BersinBio, Guangzhou, China) according to the manufacturer's protocol. The hepatocytes were collected, lysed, and sonicated. C-1 magnetic beads were incubated with circSOD2 probes at 25 °C for 2 hours to create probe-coated beads. These probe-coated beads were left in contact with the cell lysates to pull down circSOD2 at 4 °C overnight. RNA complexes with the beads were extracted using the RNeasy Mini kit (QIAGEN, Dusseldorf, Germany) and analyzed by qRT-PCR.
HEK-293T cells were seeded onto 96-well plates and cultured to reach 60%-70% confluence before transfection. Luciferase reporter plasmids and microRNAs (miRNAs) mimics were generated by GeneChem (Shanghai, China). Wild-type or mutant circSOD2 and miR-29b fragments were inserted into the Xba1 restriction site of the firefly luciferase-Renilla luciferase vector (hFLuc-XbaL-hRLuc) to generate plasmids (Luc-circSOD2 WT or Luc-circSOD2 Mut) and (Luc-miR-29b WT or Luc-miR-29b Mut). The cells were co-transfected with these luciferase reporter plasmids and different miRNA mimics using PEI transfection reagents (Sigma-Aldrich, St. Louis, MO, United States). After 48 hours, luciferase activity was evaluated using a Luciferase Assay Reagent (Yeason, Shanghai, China). Relative luciferase activity was determined as the ratio of firefly to Renilla luciferase activity.
Six-week-old Sprague Dawley rats (250 ± 50 g) were randomly distributed into different groups: Blank (Con), overexpression (AD-circSOD2), overexpression control (AD-NC), knockdown (Sh-circSOD2), knockdown control (Sh-NC) rescue (Sh-circSOD2 + antagomiR-29b), and rescue control (Sh-circSOD2 + antagomiR-NC). After anesthesia, right-side full-thickness critical-size calvarial defects (5 mm diameter) were created using a fine surgical burr under copious sterile saline irrigation. BMSCs were infected with an adenovirus to stably overexpress circSOD2 or lentivirus to stably knockdown circSOD2 and then incubated in an osteogenic medium for 7 days. Differentiated BMSCs (1 × 106 cells/mL) were seeded onto collagen I hydrogel. The rat calvarial defects were injected with 8 mL collagen I hydrogel with differentiated BMSCs. After 8 weeks, the animals were euthanized using CO2 asphyxiation followed by cervical dislocation. The calvarial samples were fixed in paraformaldehyde and scanned using a high-resolution μCT (Skyscan, Aartselaar, Belgium), and in vivo bone formation was analyzed via histomorphometry.
The data are presented as mean ± SD. Statistical analyses were performed using Student’s t-test unless otherwise specified. A P value < 0.05 was considered statistically significant. Each experiment was independently repeated at least three times.
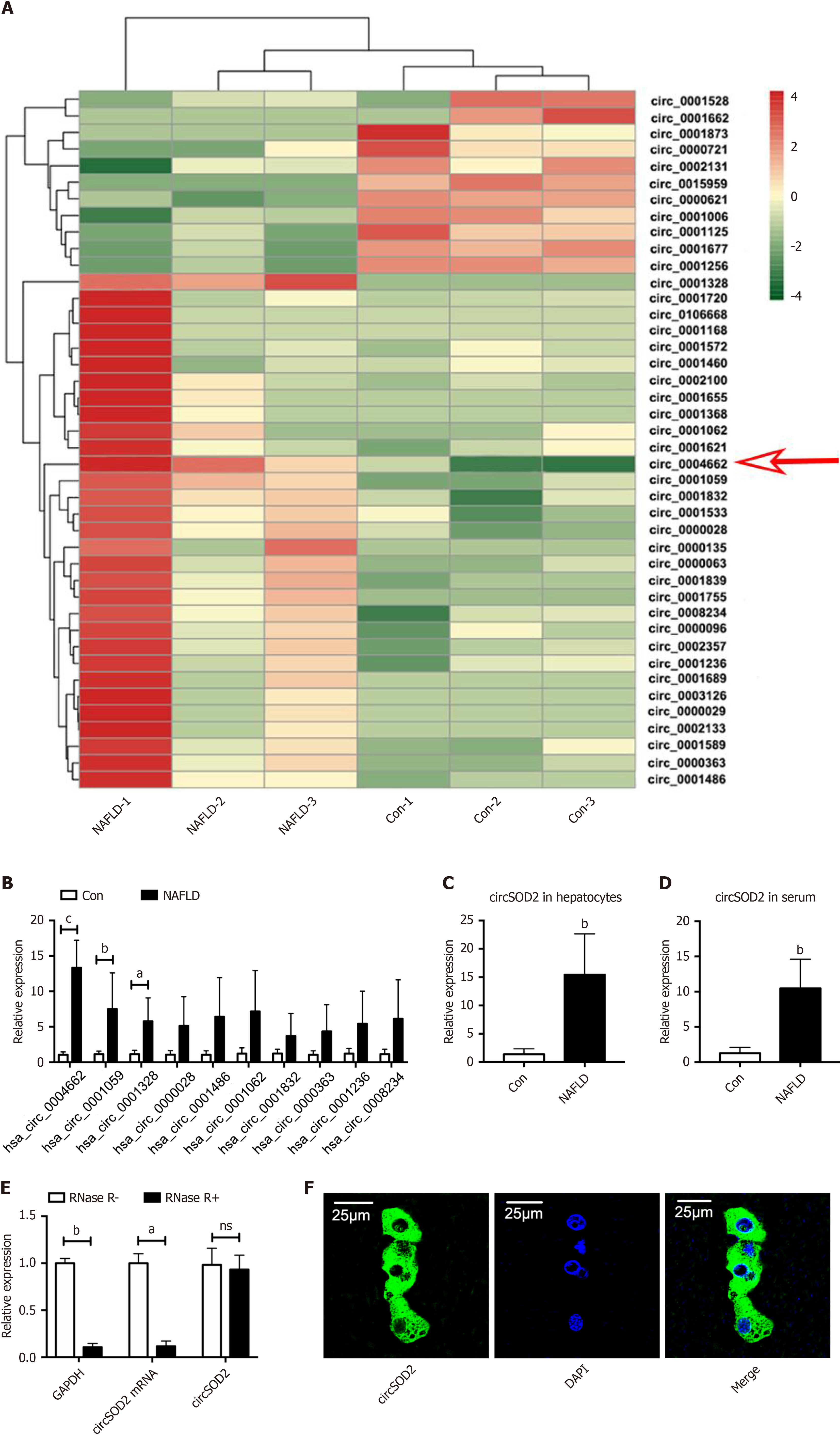
To establish a circRNA profiling database, we first performed RNA sequencing on liver tissues from three patients with MAFLD and three control subjects. A heat map revealed 42 differentially expressed circRNAs between the MAFLD and control samples, with 31 circRNAs upregulated and 11 downregulated (Figure 1A). Among the top 10 upregulated circRNAs confirmed by qRT-PCR, circSOD2 (hsa_circ_0004662) shown the highest upregulation in MAFLD liver samples (Figure 1B). Additionally, circSOD2 was highly expressed in primary hepatocytes and serum from MAFLD subjects compared to controls (Figure 1C and D). Thus, circSOD2 was selected as our target circRNA.
Previous studies have demonstrated that circSOD2 is derived from SOD2 pre-mRNA and contains three exons[22]. Compared to linear RNA, circSOD2 exhibited greater resistance to degradation by RNase R (Figure 1E), indicating enhanced stability. Moreover, we observed that circSOD2 was predominantly located in the cytoplasm according to the FISH assay (Figure 1F).
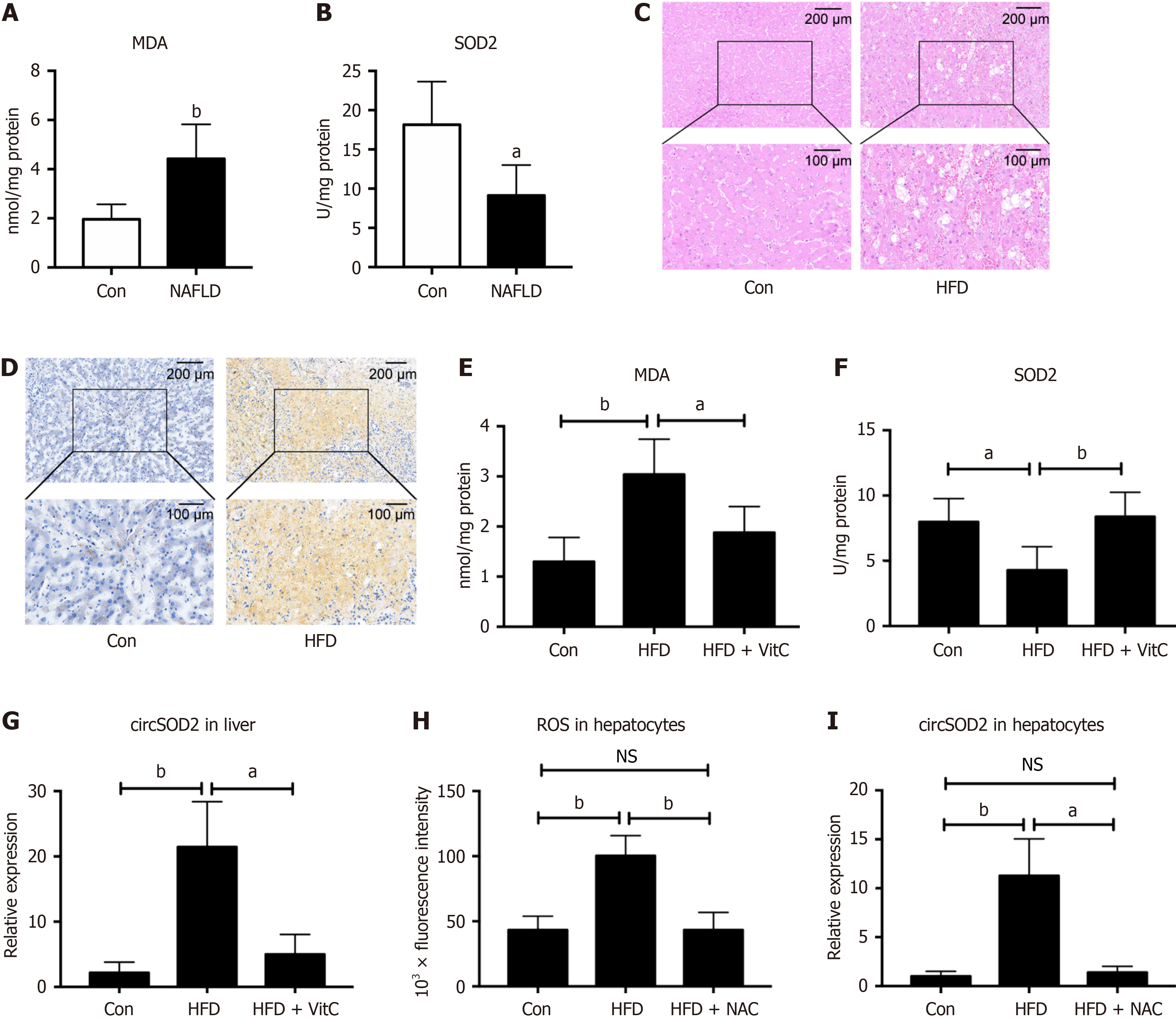
Thereafter, we explored the reason why circSOD2 expression was upregulated in NAFLD that is closely associated with increased oxidative stress. We examined the level of lipid peroxidation metabolite malondialdehyde (MDA) and an
Intracellular ROS levels were also assessed in primary mouse hepatocytes. We found that ROS levels in HFD hepatocytes were increased compared to that in control hepatocytes, while antioxidant N-acetyl L-cysteine (NAC) pretreatment abrogated the increase of ROS levels in HFD hepatocytes (Figure 2H). Correspondingly, circSOD2 expression in HFD hepatocytes was also increased compared to that in control hepatocytes, while NAC pretreatment abrogated the increase of circSOD2 expression in HFD hepatocytes (Figure 2I). Collectively, these findings suggest that circSOD2 expression is induced by oxidative stress and attenuated by antioxidants.
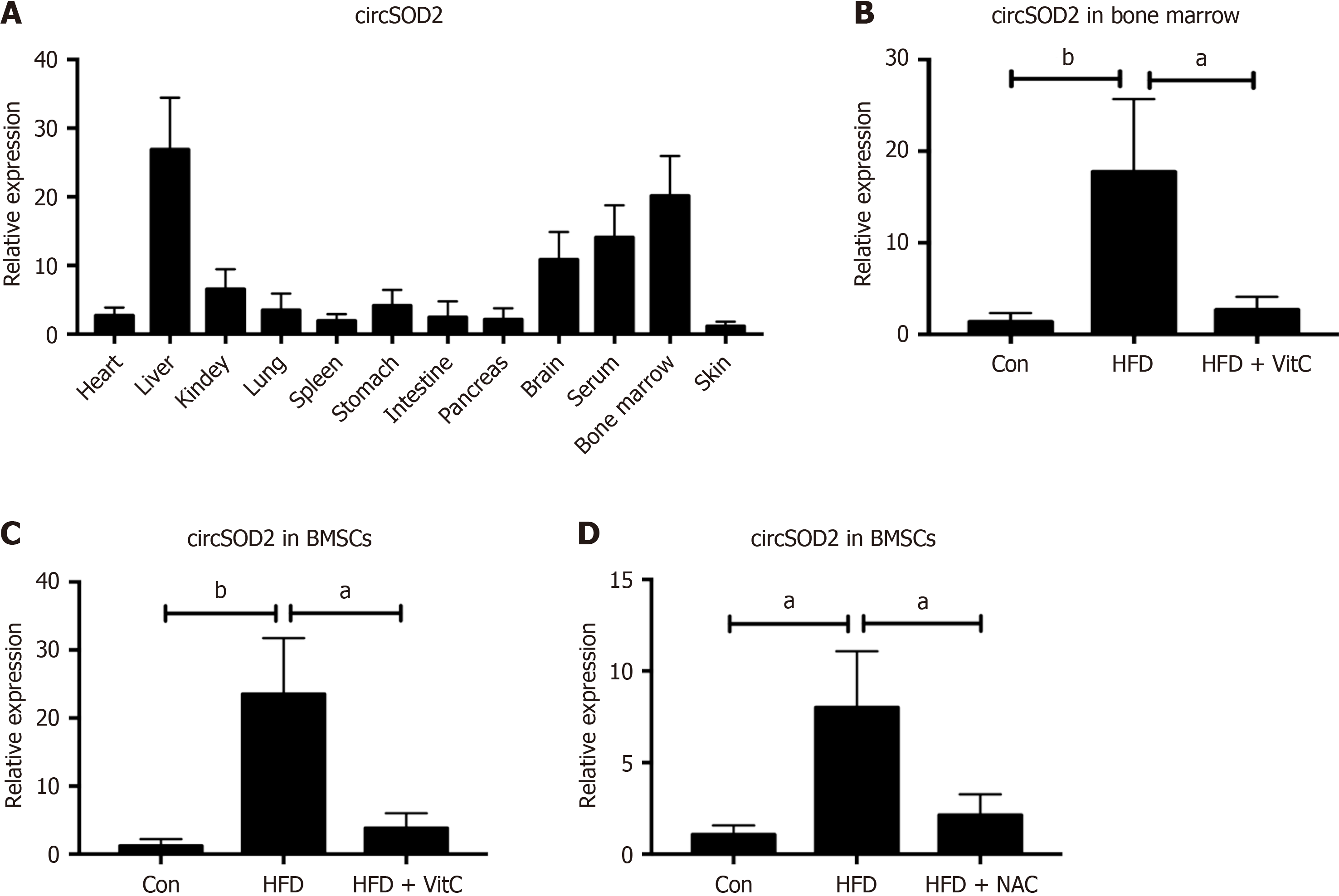
circSOD2 expression was analyzed across various organs and tissues, revealing its predominant expression in the liver, serum, and bone marrow of HFD mice (Figure 3A). CircSOD2 expression in bone marrow and BMSCs of HFD mice was significantly higher than that in bone marrow and BMSCs of normal diet mice. However, supplements with Vit C abrogated the increase of circSOD2 expression in bone marrow and BMSCs of HFD mice (Figure 3B and C). To investigate whether hepatocytes deliver circSOD2 to BMSCs, circSOD2 expression was measured in BMSCs co-cultured with hepatocytes. BMSCs co-cultured with HFD hepatocytes exhibited elevated circSOD2 Levels compared to control hepatocytes. However, HFD hepatocytes pretreated with NAC abrogated the increase of circSOD2 expression in co-culturedBMSCs (Figure 3D).
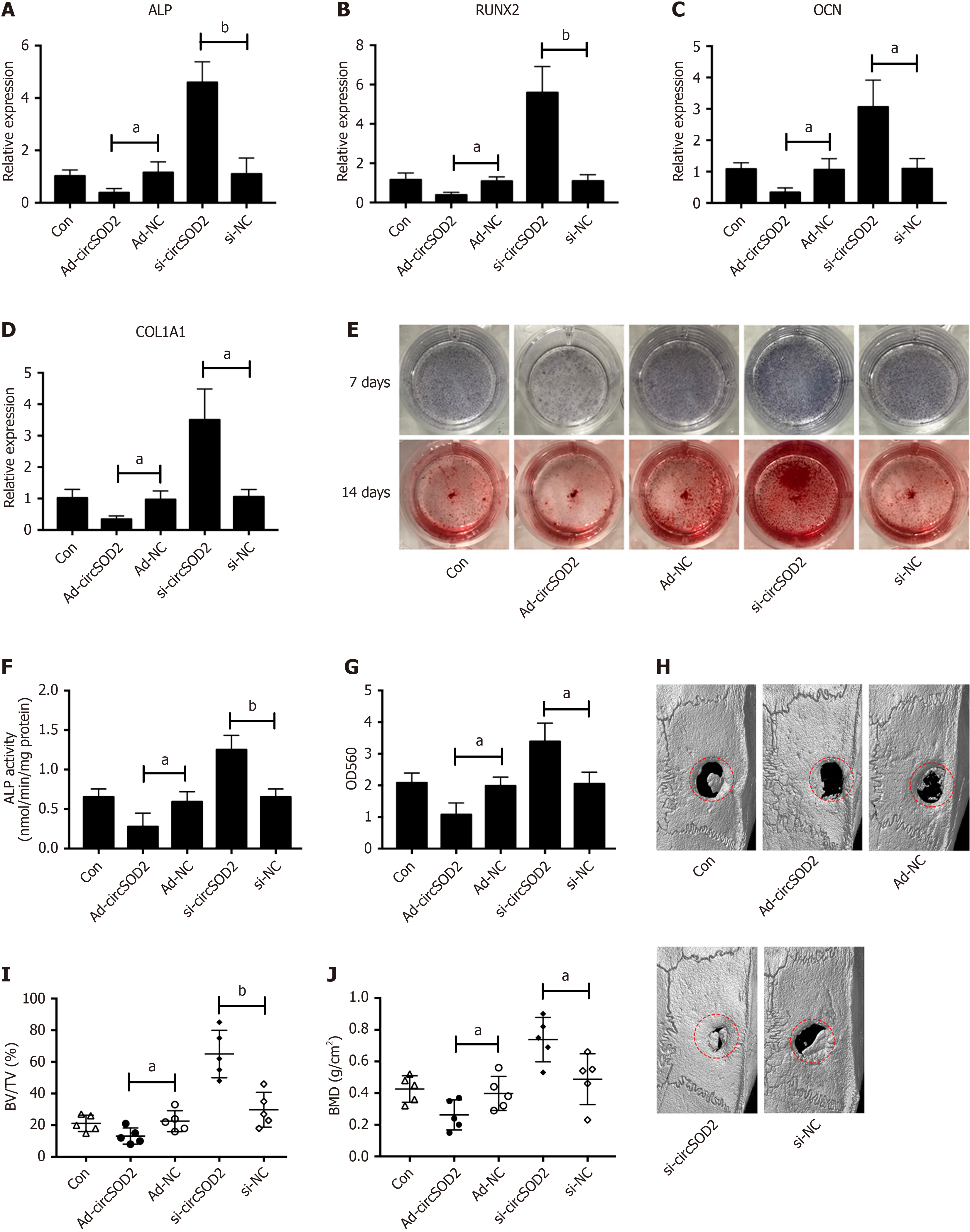
Given the upregulation of circSOD2 in BMSCs from HFD mice, we investigated its role in regulating osteogenic differentiation. BMSCs were incubated in an osteogenic medium following transfection with AD-circSOD2 or si-circSOD2. We found that osteogenic markers, including ALP, runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and collagen type 1 alpha 1 (COL1A1), were suppressed by circSOD2 overexpression and enhanced by circSOD2 knockdown during osteogenic differentiation of BMSCs (Figure 4A-D). Similarly, ALP activity, as evidenced by ALP staining and calcium deposition, assessed by Alizarin Red staining, followed the same trend (Figure 4E-G). Furthermore, a rat model of calvarial critical-size defects was used to confirm the effect of circSOD2 on osteogenesis. Micro-computed tomography (micro-CT) revealed that circSOD2 overexpression inhibited, while its silencing enhanced in vivo bone formation in rats (Figure 4H). Further analysis showed that calvarial trabecular bone volume per tissue volume (BV/TV) and BMD decreased in response to circSOD2 overexpression and increased in response to circSOD2 knockdown (Figure 4I and J).
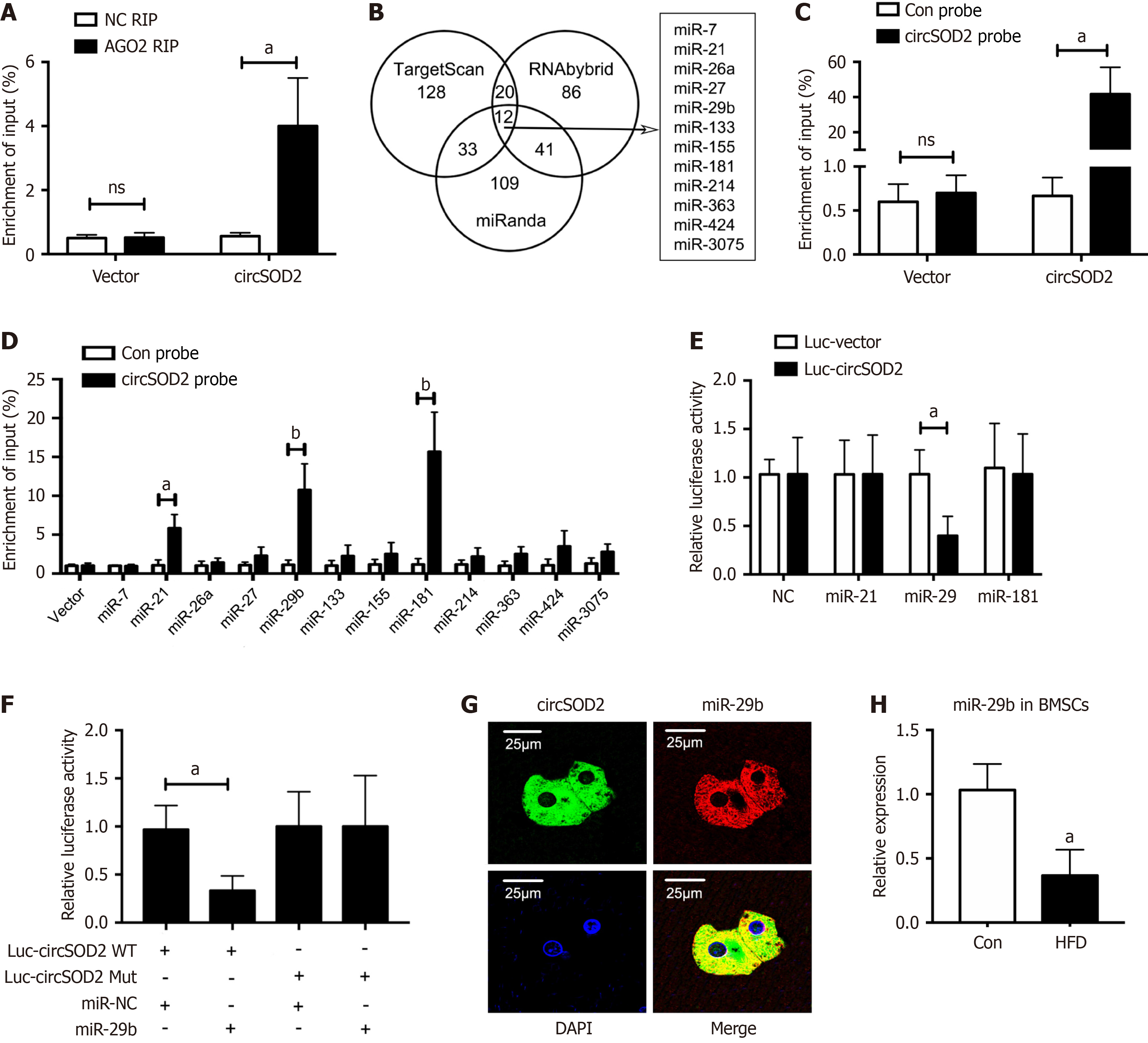
Next, we investigated whether circSOD2 could bind to miRNAs. We observed that circSOD2 was notably enriched in RIP with an antibody against argonaute 2 (AGO2) that binds miRNAs (Figure 5A), indicating that circSOD2 may function as a miRNA sponge. By cross-referencing the TargetScan, RNAbybrid and miRanda databases, we identified 12 candidate miRNAs as potential targets of circSOD2 (Figure 5B). An antisense RNA probe specific for circSOD2 was designed and validated (Figure 5C). Three miRNAs were identified to be associated with circSOD2 using RAP (Figure 5D). Among these, only miR-29b mimic showed a significant reduction in relative luciferase activity, indicating miR-29b binding to circSOD2 (Figure 5E). Therefore, miR-29b was selected for further analysis. Subsequently, the dual-luciferase reporter assay showed that transfection of miR-29b mimic led to a significant reduction in relative luciferase activity of wild-type circSOD2 (Figure 5F). Moreover, FISH assay indicated the co-localization of circSOD2 and miR-29bin the cytoplasm (Figure 5G). In contrast with circSOD2 expression, miR-29b expression was significantly decreased in BMSCs from HFD mice compared to controls (Figure 5H). Collectively, these findings suggest that circSOD2 serves as a sponge for the downstream target miR-29b.
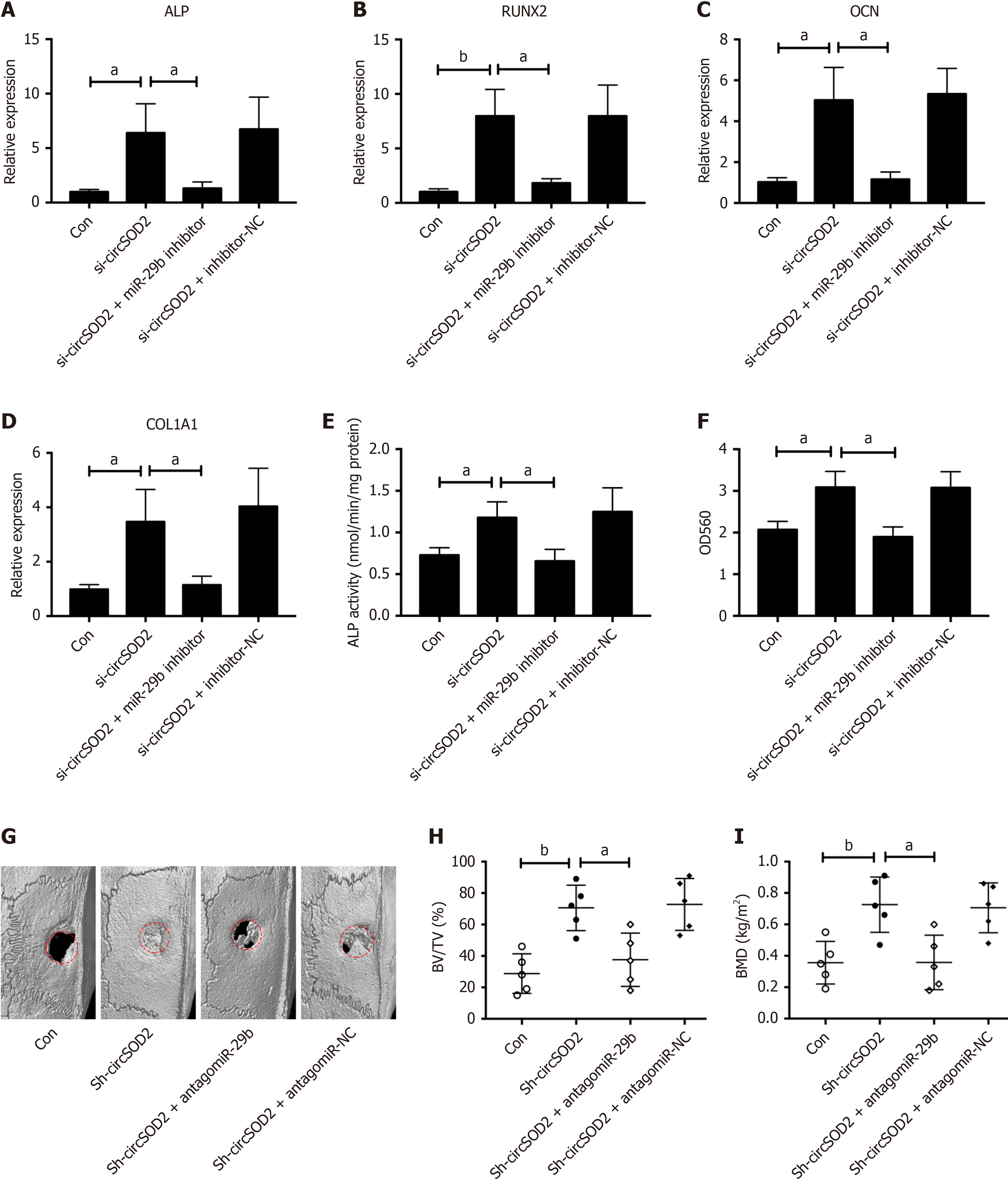
Given the pivotal role of miR-29b, a downstream target of circSOD2, in osteogenic differentiation[23] and bone formation[24], we hypothesized that circSOD2 exerts its inhibitory effects on osteogenic differentiation and bone formation through miR-29b. To test the hypothesis, BMSCs were incubated in an osteogenic medium after co-transfection with si-circSOD2 with or without miR-29b inhibitor. CircSOD2 knockdown significantly stimulated osteogenic markers, including ALP, RUNX2, OCN, and COL1A1, however, miR-29b silencing reversed the stimulatory effect of circSOD2 knockdown on these markers (Figure 6A-D). Likewise, the change pattern of ALP activity and calcium deposition was consistent with osteogenic markers in response to miR-29b inhibition (Figure 6E and F). Furthermore, micro-CT demonstrated that miR-29b silencing abrogated the stimulatory effect of circSOD2 knockdown on in vivo bone formation in rats (Figure 6G). In detail, miR-29b silencing abrogated the stimulatory effect of circSOD2 knockdown on BV/TV and BMD (Figure 6H and I). Taken together, these findings suggest that circSOD2 inhibits osteogenesis via miR-29b.
The liver, as the central organ of metabolism, communicates with other organs through endocrine signaling in the whole body. The crosstalk between the liver with other tissues such as adipose tissue, heart, and brain has been extensively studied[25,26]. However, the interaction between the liver and bone remains poorly understood. To our knowledge, this study is the first to explore the liver-bone crosstalk from the perspective of circRNAs. We screened and verified differentially expressed circRNAs in MAFLD livers, focusing on the upstream and downstream mechanisms of circSOD2 upregulation during osteogenesis. Our finding revealed that circSOD2 upregulation was induced by oxidative stress in hepatocytes. Moreover, circSOD2 was delivered from hepatocytes to BMSCs via blood circulation. Furthermore, circSOD2 inhibited osteogenic differentiation of BMSCs and bone formation by sponging miR-29b.
Dysregulated circRNAs have been implicated in various diseases, including cancer and MAFLD[27]. Due to high stability and easy detection, they are increasingly investigated as promising disease biomarkers and therapeutic targets[28]. Recent studies have revealed alterations in circRNA expression in patients and animal models of MAFLD, primarily focusing on their role in MAFLD[29-32]. However, their impact on the metabolism of extrahepatic tissues, including bone, remains largely unexplored.
CircSOD2, with a mature spliced sequence of 462 bp, is located on the reverse strand of human chromosome 6 and is ubiquitously expressed in many tissues and cells. Recent evidence has shown thatcircSOD2 was highly expressed in smooth muscle cells[22], M1 macrophages[33], osteoarthritic cartilage[34], and hepatocellular carcinoma tissues[35]. Consistent with these findings, our results showed that circSOD2 was highly expressed in hepatocytes, liver, serum, and bone marrow. Notably, circSOD2 expression was upregulated in the liver and serum of patients with MAFLD and HFD mice compared to controls. Given its stable expression and detectability in blood, our results suggest that circSOD2 may represent a promising and noninvasive biomarker for MAFLD, offering a viable alternative to puncture liver biopsy.
Extensive studies have shown that oxidative stress is a pivotal factor in the pathophysiology of MAFLD. Chronic inflammation and lipid metabolism disorders are closely related to an imbalance between oxidants and antioxidants, leading to lipid peroxidation and mitochondrial dysfunction in MAFLD[36]. Our findings revealed increased MDA levels and decreased SOD2 Levels in patients with MAFLD and HFD mice. Further investigation into the upstream regulation of circSOD2 revealed that its expression was upregulated by oxidative stress but was mitigated by Vit C or NAC pretreatment. These findings align with growing interest in antioxidants and antioxidative drugs as potential strategies to mitigate MAFLD development and progression.
Clinical evidence increasingly supports a strong association between low BMD or osteoporosis and MAFLD in both male and female adults[9,11,37,38]. This relationship is not restricted to adults, highlighted by a recent meta-analysis reporting that MAFLD is significantly linked to reduced whole-body BMD in children and adolescents[39]. Despite this evidence, the causal relationship is still not defined, not to mention molecular mechanism. Interestingly, we found that hepatocytes delivered circSOD2 to BMSCs, as confirmed through cell co-culture experiments, indicating that circSOD2 mediates the crosstalk between the liver and bone via endocrine signaling. Gain- and loss-of-function analyses further revealed that circSOD2 overexpression inhibited osteogenic differentiation of BMSCs and bone formation, whereas circSOD2 silencing enhanced these processes. Our findings provide cellular and molecular evidence to support the mainstream view that MAFLD is a significant risk factor for low BMD or osteoporosis. Furthermore, our results suggest that targeting circSOD2 represents a promising therapeutical approach to prevent the deleterious effects of MAFLD on bone formation and prevent osteoporosis in patients with severe MAFLD.
CircRNAs regulate cell behavior through diverse mechanisms, such as interaction with miRNAs or proteins and modulation of transcription and translation[17,40]. miRNAs, a class of small single-stranded non-coding RNAs, regulate gene expression through mRNA degradation or translational inhibition[41]. Several studies have reported that circSOD2 acts as a sponge for several miRNAs, such as miR-206[22], miR-224-5p[34], miR-502-5p[35], and miR-1296[33]. Similarly, we found an interaction between circSOD2 and miR-29b using a luciferase reporter assay and observed their co-localization in the cytoplasm, as evidenced by the FISH assay. These findings indicate that circSOD2 functions as a competitive RNA, suppressing the activity of miR-29b. Previous studies, including our own, have shown that miRNAs play a crucial role in the osteogenic differentiation of MSCs[7,42]. Furthermore, in vitro and in vivo rescue experiments indicated that circSOD2 inhibited the osteogenic differentiation of BMSCs and bone formation through miR-29b. Our findings were consistent with previous reports that miR-29b promoted osteoblast differentiation by targeting anti-osteogenic factors[43] and biomaterials-mediated intracellular delivery of miRNA-29b enhanced osteogenic differentiation of MSCs[23] and bone regeneration[2]. Given that miR-29b has been identified to regulate the Wnt/β-catenin pathway in prior research[44,45], we speculated that the circSOD2/miR-29b axis also regulates osteogenic differentiation via the Wnt/β-catenin pathway.
The present study had certain limitations. Firstly, the downstream targets and signaling pathways of miR-29b were not investigated. Secondly, the expression of miR-29b in human samples and its relationship with MAFLD was not investigated. Thirdly, the therapeutic effect of targeting circSOD2 was not investigated using an animal model of osteoporosis. Finally, we were unable to establish an animal model of MAFLD simultaneously accompanied by osteoporosis to study this relationship.
In summary, this study demonstrates a novel mechanism underlying the crosstalk between the liver and bone. We showed thatcircSOD2 upregulation, induced by oxidative stress in the context of MAFLD, inhibits osteogenesis by sponging miRNA-29b. These findings offer insights into the relationship between MAFLD and osteoporosis, suggesting that MAFLD may contribute to the pathogenesis of osteoporosis. Targeting circSOD2 represents a potential strategy for preventing osteoporosis in patients with MAFLD, which warrants further investigation in future studies.
| 1. | Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 2924] [Article Influence: 417.7] [Reference Citation Analysis (1)] |
| 2. | Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 459] [Article Influence: 57.4] [Reference Citation Analysis (2)] |
| 3. | Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999-2014.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2367] [Cited by in RCA: 2212] [Article Influence: 442.4] [Reference Citation Analysis (1)] |
| 4. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7544] [Article Influence: 838.2] [Reference Citation Analysis (0)] |
| 5. | Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 805] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 6. | Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 806] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 7. | Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8:212-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 470] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 8. | Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 1473] [Article Influence: 245.5] [Reference Citation Analysis (0)] |
| 9. | Li M, Xu Y, Xu M, Ma L, Wang T, Liu Y, Dai M, Chen Y, Lu J, Liu J, Bi Y, Ning G. Association between nonalcoholic fatty liver disease (NAFLD) and osteoporotic fracture in middle-aged and elderly Chinese. J Clin Endocrinol Metab. 2012;97:2033-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Upala S, Jaruvongvanich V, Wijarnpreecha K, Sanguankeo A. Nonalcoholic fatty liver disease and osteoporosis: a systematic review and meta-analysis. J Bone Miner Metab. 2017;35:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Shen Z, Cen L, Chen X, Pan J, Li Y, Chen W, Yu C. Increased risk of low bone mineral density in patients with non-alcoholic fatty liver disease: a cohort study. Eur J Endocrinol. 2020;182:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Chen HJ, Yang HY, Hsueh KC, Shen CC, Chen RY, Yu HC, Wang TL. Increased risk of osteoporosis in patients with nonalcoholic fatty liver disease: A population-based retrospective cohort study. Medicine (Baltimore). 2018;97:e12835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Pan B, Cai J, Zhao P, Liu J, Fu S, Jing G, Niu Q, Li Q. Relationship between prevalence and risk of osteoporosis or osteoporotic fracture with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Osteoporos Int. 2022;33:2275-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Su YH, Chien KL, Yang SH, Chia WT, Chen JH, Chen YC. Nonalcoholic Fatty Liver Disease Is Associated With Decreased Bone Mineral Density in Adults: A Systematic Review and Meta-Analysis. J Bone Miner Res. 2023;38:1092-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Liu CX, Chen LL. Circular RNAs: Characterization, cellular roles, and applications. Cell. 2022;185:2016-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 563] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 16. | Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3273] [Cited by in RCA: 3154] [Article Influence: 525.7] [Reference Citation Analysis (0)] |
| 17. | Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 1019] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 18. | Mazziotta C, Badiale G, Cervellera CF, Tognon M, Martini F, Rotondo JC. Regulatory mechanisms of circular RNAs during human mesenchymal stem cell osteogenic differentiation. Theranostics. 2024;14:143-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 19. | Chen Z, Lin W, Zhao S, Mo X, Wen Z, Cheung WH, Fu D, Chen B. Identification of circRNA Expression Profiles in BMSCs from Glucocorticoid-Induced Osteoporosis Model. Stem Cells Int. 2022;2022:3249737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Zeng Q, Liu CH, Wu D, Jiang W, Zhang N, Tang H. LncRNA and circRNA in Patients with Non-Alcoholic Fatty Liver Disease: A Systematic Review. Biomolecules. 2023;13:560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Zeng Q, Liu CH, Ampuero J, Wu D, Jiang W, Zhou L, Li H, Bai L, Romero-Gómez M, Tang H. Circular RNAs in non-alcoholic fatty liver disease: Functions and clinical significance. RNA Biol. 2024;21:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Mei X, Cui XB, Li Y, Chen SY. CircSOD2: A Novel Regulator for Smooth Muscle Proliferation and Neointima Formation. Arterioscler Thromb Vasc Biol. 2021;41:2961-2973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Suh JS, Lee JY, Choi YS, Chung CP, Park YJ. Peptide-mediated intracellular delivery of miRNA-29b for osteogenic stem cell differentiation. Biomaterials. 2013;34:4347-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Qin H, Ji Y, Li G, Xu X, Zhang C, Zhong W, Xu S, Yin Y, Song J. MicroRNA-29b/graphene oxide-polyethyleneglycol-polyethylenimine complex incorporated within chitosan hydrogel promotes osteogenesis. Front Chem. 2022;10:958561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 25. | Cao Y, Wang Y, Zhou Z, Pan C, Jiang L, Zhou Z, Meng Y, Charugundla S, Li T, Allayee H, Seldin MM, Lusis AJ. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science. 2022;377:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 26. | Zhao J, Lei H, Wang T, Xiong X. Liver-bone crosstalk in non-alcoholic fatty liver disease: Clinical implications and underlying pathophysiology. Front Endocrinol (Lausanne). 2023;14:1161402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 27. | Yepmo M, Potier JB, Pinget M, Grabarz A, Bouzakri K, Dumond Bourie A. Discussing the role of circular RNA in the pathogenesis of non-alcoholic fatty liver disease and its complications. Front Endocrinol (Lausanne). 2022;13:1035159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 28. | Nemeth K, Bayraktar R, Ferracin M, Calin GA. Non-coding RNAs in disease: from mechanisms to therapeutics. Nat Rev Genet. 2024;25:211-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 344] [Article Influence: 344.0] [Reference Citation Analysis (0)] |
| 29. | Zhao Q, Liu J, Deng H, Ma R, Liao JY, Liang H, Hu J, Li J, Guo Z, Cai J, Xu X, Gao Z, Su S. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell. 2020;183:76-93.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 30. | Yuan X, Diao J, Du A, Wen S, Zhou L, Pan Y. Circular RNA expression profiles and features in NAFLD mice: a study using RNA-seq data. J Transl Med. 2020;18:476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Chen X, Tan QQ, Tan XR, Li SJ, Zhang XX. Circ_0057558 promotes nonalcoholic fatty liver disease by regulating ROCK1/AMPK signaling through targeting miR-206. Cell Death Dis. 2021;12:809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Xu ZX, Li JZ, Li Q, Xu MY, Li HY. CircRNA608-microRNA222-PINK1 axis regulates the mitophagy of hepatic stellate cells in NASH related fibrosis. Biochem Biophys Res Commun. 2022;610:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 33. | Qu B, Liu J, Peng Z, Xiao Z, Li S, Wu J, Li S, Luo J. CircSOD2 polarizes macrophages towards the M1 phenotype to alleviate cisplatin resistance in gastric cancer cells by targeting the miR-1296/STAT1 axis. Gene. 2023;887:147733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Li H, Cao Y, Chang C, Huang W, Su S, Peng Z, Zhang J. Knockdown of circSOD2 ameliorates osteoarthritis progression via the miR-224-5p/PRDX3 axis. J Orthop Surg Res. 2023;18:432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Zhao Z, Song J, Tang B, Fang S, Zhang D, Zheng L, Wu F, Gao Y, Chen C, Hu X, Weng Q, Yang Y, Tu J, Ji J. CircSOD2 induced epigenetic alteration drives hepatocellular carcinoma progression through activating JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res. 2020;39:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 36. | Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants (Basel). 2021;10:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 295] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 37. | Mosca A, Fintini D, Scorletti E, Cappa M, Paone L, Zicari AM, Nobili V, Byrne CD. Relationship between non-alcoholic steatohepatitis, PNPLA3 I148M genotype and bone mineral density in adolescents. Liver Int. 2018;38:2301-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Moon SS, Lee YS, Kim SW. Association of nonalcoholic fatty liver disease with low bone mass in postmenopausal women. Endocrine. 2012;42:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Mantovani A, Gatti D, Zoppini G, Lippi G, Bonora E, Byrne CD, Nobili V, Targher G. Association Between Nonalcoholic Fatty Liver Disease and Reduced Bone Mineral Density in Children: A Meta-Analysis. Hepatology. 2019;70:812-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4631] [Cited by in RCA: 6050] [Article Influence: 504.2] [Reference Citation Analysis (0)] |
| 41. | Shang R, Lee S, Senavirathne G, Lai EC. microRNAs in action: biogenesis, function and regulation. Nat Rev Genet. 2023;24:816-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 399] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 42. | Li L, Qi Q, Luo J, Huang S, Ling Z, Gao M, Zhou Z, Stiehler M, Zou X. FOXO1-suppressed miR-424 regulates the proliferation and osteogenic differentiation of MSCs by targeting FGF2 under oxidative stress. Sci Rep. 2017;7:42331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676-15684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 468] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 44. | Xia T, Dong S, Tian J. miR29b promotes the osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue via the PTEN/AKT/βcatenin signaling pathway. Int J Mol Med. 2020;46:709-717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Shin J, Shin Y, Oh SM, Yang H, Yu WJ, Lee JP, Huh SO, Lee SH, Suh YH, Chung S, Kim HS. MiR-29b controls fetal mouse neurogenesis by regulating ICAT-mediated Wnt/β-catenin signaling. Cell Death Dis. 2014;5:e1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |