Published online Mar 7, 2025. doi: 10.3748/wjg.v31.i9.102059
Revised: November 29, 2024
Accepted: January 21, 2025
Published online: March 7, 2025
Processing time: 133 Days and 20.3 Hours
Histoplasmosis is a rare infectious condition with mainly pulmonary involvement which is generally self-limiting in immunocompetent individuals. Its manife
A 43-year-old Chinese man with intermittent fever, malaise, jaundice and extreme hepatomegaly for more than 40 days was admitted to the Second Xiang-ya Hospital. The patient was immunocompetent and lacked a definitive history of exposure. His condition deteriorated to liver failure, and he promptly underwent liver transplantation to ensure survival. One year later, the patient presented with severe gastrointestinal symptoms, including fever, abdominal pain, and diarrhea. Subsequently, tissue samples acquired via gastrointestinal endoscopy were subjected to pathological examination and next-generation sequencing analysis. Through a comprehensive amalgamation of clinical presentation, biopsy pathology, and next-generation sequencing analysis, the patient was ultimately diagnosed with disseminated hepatic histoplasmosis. The patient achieved complete recovery after 6 months of voriconazole treatment.
In patients with chronic-hepatitis-B having atypical symptoms, histoplasmosis can be a differential diagnosis. Voriconazole is effective in treating histoplasmosis.
Core Tip: Disseminated histoplasmosis in immunocompetent populations presenting solely as liver involvement and progressing to liver failure is rare, and there is no reported case of histoplasmosis cured by liver transplantation. Voriconazole is commonly used for fungal infections, but its experience in treating histoplasmosis is limited. In our report, we detail the case of the world’s first patient to receive liver transplantation surgery for liver failure caused by hepatic histoplasmosis, characterized by a complex and varied progression. After a comprehensive analysis, the patient was ultimately diagnosed and achieved complete recovery with the surgery and voriconazole.
- Citation: Li CX, Chen HD, Kashif S, Xie B, Luo J, Li T. Disseminated liver histoplasmosis in an immunocompetent individual cured by liver transplantation and voriconazole from China: A case report. World J Gastroenterol 2025; 31(9): 102059
- URL: https://www.wjgnet.com/1007-9327/full/v31/i9/102059.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i9.102059
Histoplasmosis is a systemic fungal infection mainly caused by the respiratory transmission of Histoplasma capsulatum. The microspores of which can be found mainly in warm, moist, nitrogen-rich soils[1]. Histoplasmosis is usually self-limiting in immunocompetent populations. However, in immunocompromised people, such as those infected with human immunodeficiency virus, organ transplant recipients, and patients on long-term immunosuppressive drugs, the disease can develop into life-threatening progressive disseminated histoplasmosis. Histoplasmosis is prevalent in the midwestern United States, mainland of Latin America, and Africa. In recent years, the number of reported cases of histoplasmosis in China has risen. A retrospective study of 582 histoplasmosis cases in mainland China (2000-2024) revealed a southward geographic shift, aligning with a prior review of 300 cases (1990-2011)[2]. Accurately tracing the origin of histoplasmosis remains challenging due to the high mobility of the population in China, as most cases lack detailed information on residency or travel history. On available information, histoplasmosis is almost exclusively distributed in the Yangtze River basin and provinces to its south, with Yunnan being a possible hot spot[3]. However, these cases are mostly sporadic and are concentrated in provinces along the Yangtze River, such as the Yunnan, Hunan, and Hubei provinces[4].
Disseminated histoplasmosis originates from pulmonary infections. The clinical presentation is nonspecific and highly variable, with approximately 68.1% of disseminated histoplasmosis infections involving the liver[4]. However, disseminated histoplasmosis with predominantly hepatic involvement is rare. It usually presents as fever, malaise, jaundice, and ascites, and can progress to chronic liver disease, cholestasis, portal hypertension, and even liver failure[5]. We report the world’s first case of subacute hepatic failure due to progressive cholestasis caused by histoplasmosis in immunocompetent host, which was treated by liver transplantation and antifungal therapy. This case provides valuable experience that may inform the treatment of similar cases in the future.
A 43-year-old man from Qiyang County, residing in Hunan Province, was admitted to the Infectious Diseases Depar
His maximum temperature reached 102 °F and subsided following antipyretic administration. Based on clinical data, physical examination findings, and investigation results described below, the case was initially diagnosed as chronic severe hepatitis secondary to hepatitis B.
The patient had hypertension for 5 years without regular monitoring or control, chronic and hepatitis B since childhood without medical intervention. The patient denies a history of diabetes mellitus, tuberculosis, typhoid fever, malaria, heart disease, cerebrovascular disease, or mental illness. No history of trauma or surgery. Denies any food or drug allergies.
The patient was a taxi driver. He denied any recent travel, surgery, or trauma history. He had a family history of hepatitis B and was born in Hengyang City, Hunan Province. He had no history of tobacco or alcohol, or long-term medication use, and denied any history of specific genetic diseases.
On general examination, hepatomegaly about 3 cm below the right costal margin with mild tenderness was observed.
A complete blood count revealed the following: Hemoglobin 95 g/L, white blood cell count 11.27 × 109/L, and platelet count 86 × 109/L. Liver function tests showed total bilirubin/direct bilirubin levels of 459.1/333.6 μmol/L with transaminase levels abnormally low. Coagulation tests indicated a prothrombin time of 20.3 seconds, an activated partial thromboplastin time of 71.0 seconds, and an international normalized ratio of 1.77. Hepatitis B serological markers were positive for hepatitis B surface antigen, hepatitis B e antibody, and hepatitis B core antibody, with a hepatitis B virus DNA level of 36.6 IU/mL. Blood tests revealed no significant inflammation, with only slight elevations in erythrocyte sedimentation rate, 3 mm/hour) and procalcitonin (2.26 ng/mL). To exclude autoimmune diseases, serum immunological tests, including anti-mitochondrial antibodies, were performed, and all returned negative. Due to the patient’s poor overall condition, coagulation disorders, and rapid disease progression, a liver biopsy was not performed to assess specific pathogenic infections. No definitive infections were identified, as sputum, blood, and ascitic fluid cultures, along with tests for glucan, Epstein-Barr virus DNA, cytomegalovirus DNA, and anti-tuberculosis antibodies, all returned negative results. The condition progressed to liver failure within 2 weeks, and the preoperative test results indicated total bilirubin/direct bilirubin levels of 469.4/324.3 μmol/L, a prothrombin time of 17.9 seconds, an activated partial thromboplastin time of 48.3 seconds, and prothrombin time activity of 35.8%.
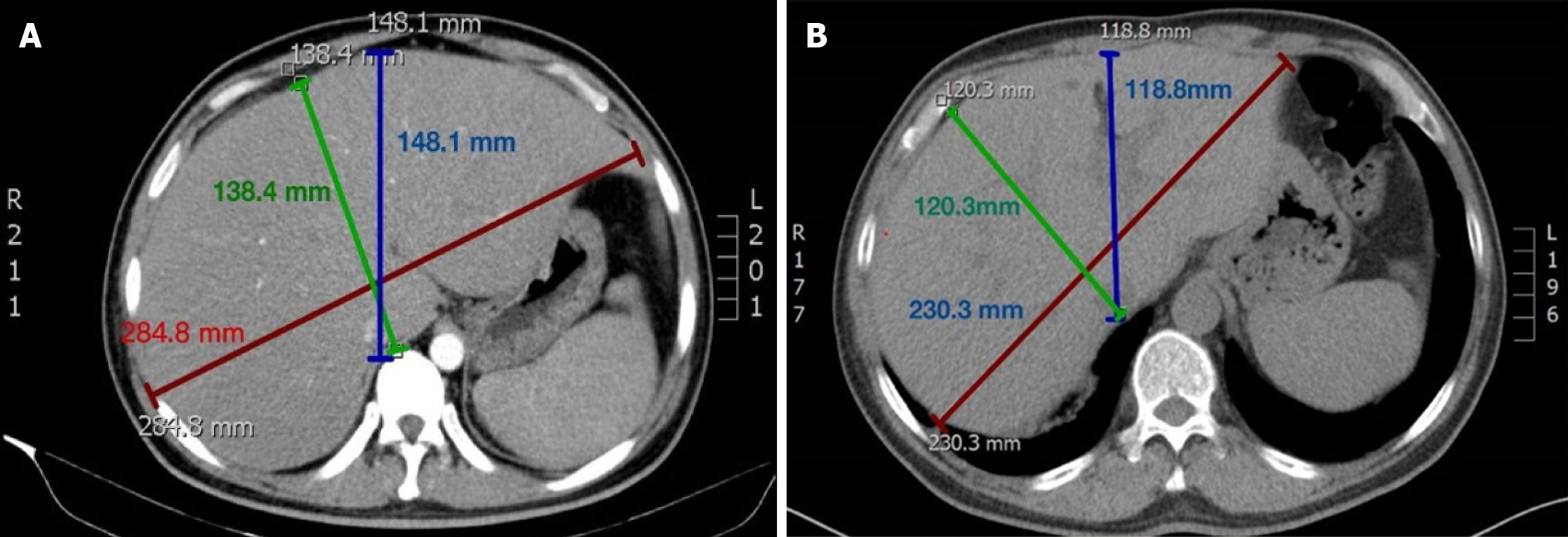
Abdominal computed tomography (CT) indicated hepatomegaly with a maximum transverse diameter of 287.0 mm at the level of the hepatoportal (Figure 1). The lung CT returned negative results.
The final diagnosis is disseminated liver histoplasmosis.
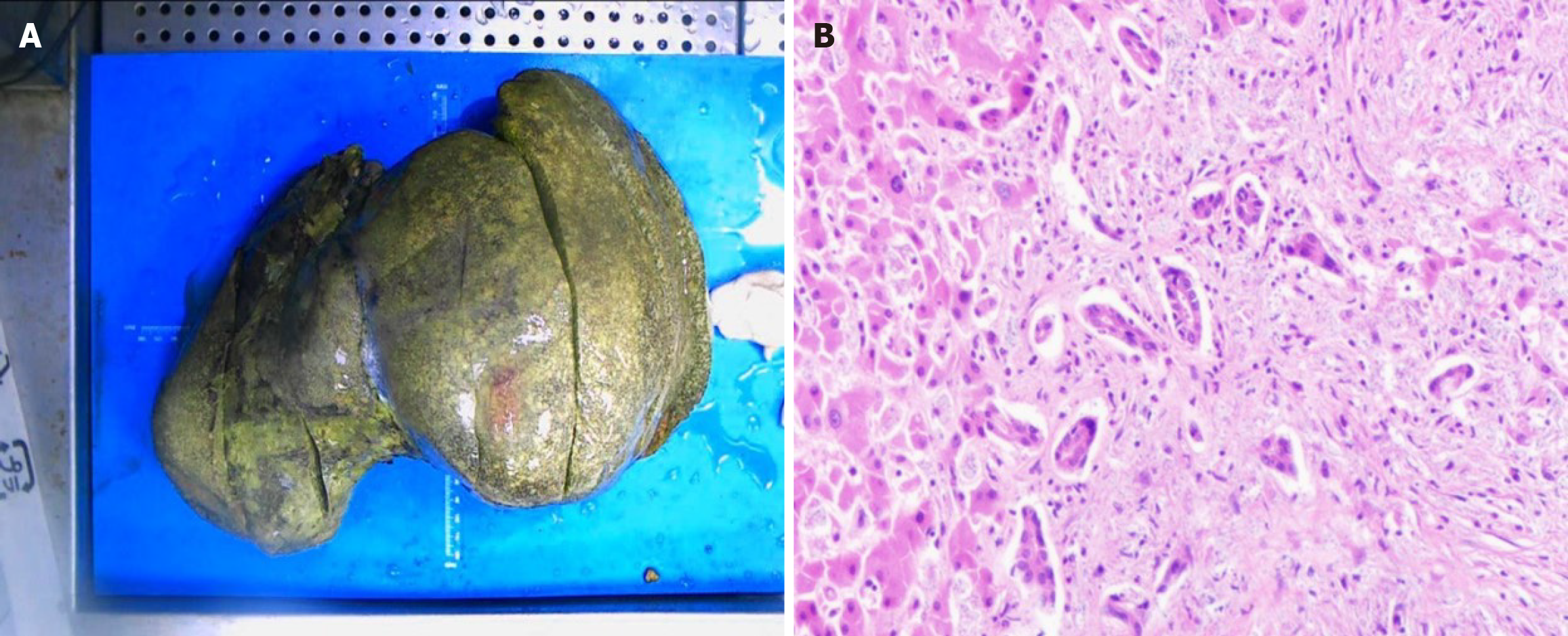
The patient was promptly started on entecavir therapy to manage the hepatitis B virus infection. Despite receiving various empirical antibiotics and undergoing multiple plasmapheresis sessions, the fever persisted, and liver function failed to improve. The condition progressed to liver failure within 2 weeks. After a comprehensive evaluation of surgical conditions and associated risks, the patient underwent in situ liver transplantation on March 19, 2021. Contrary to the typical atrophic liver seen in severe CHB, the patient’s liver was significantly enlarged (38 cm × 25 cm × 15 cm), dark in color, and covered with numerous yellow spots (Figure 2). Pathological microscopy revealed sub-massive necrosis of liver tissue, disruption of the normal hepatic lobular structure, and extensive lymphocytic infiltration.
Postoperatively, the patient received infection prophylaxis with 1000 mg meropenem every 8 hours, 50 mg cas
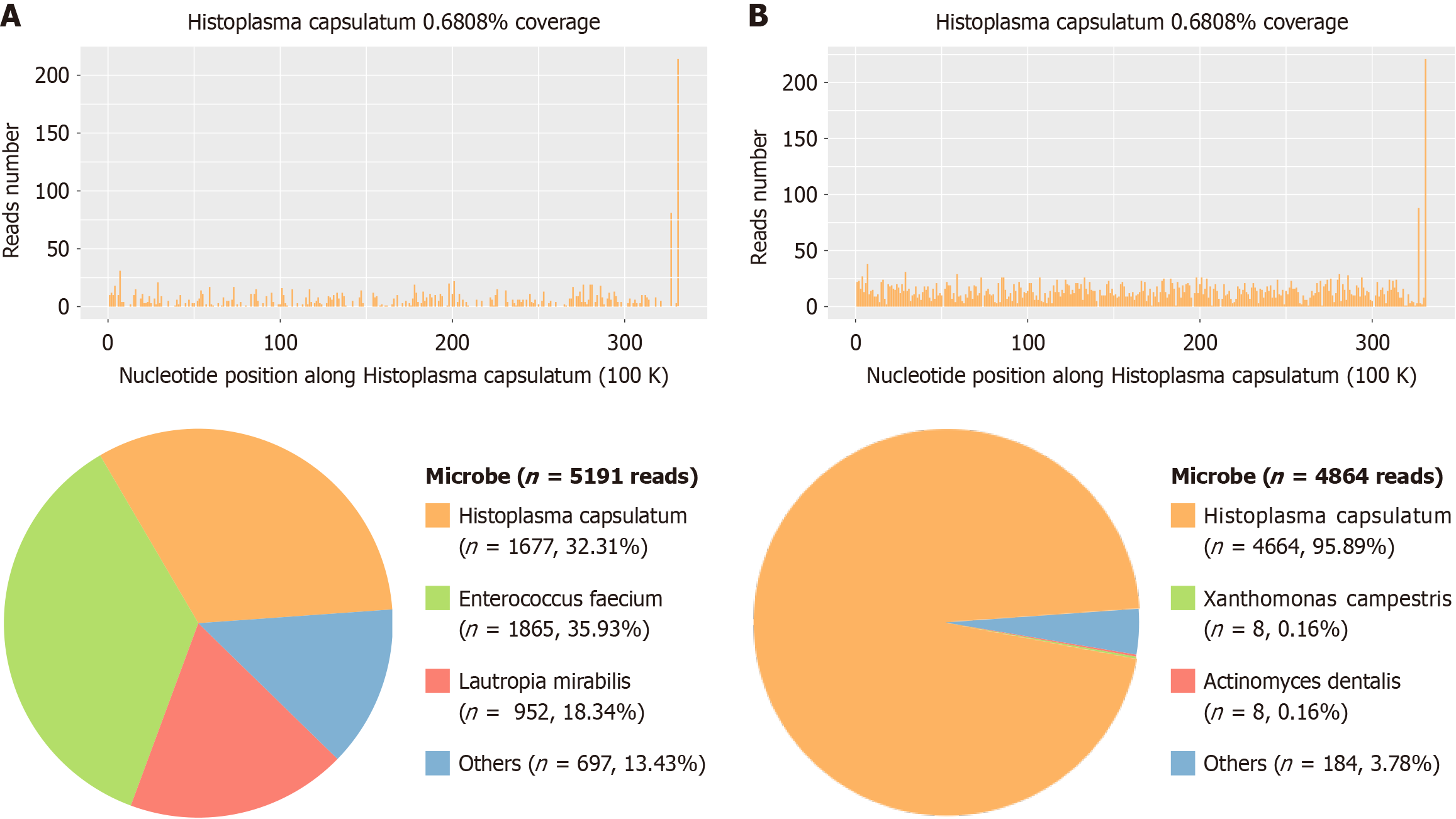
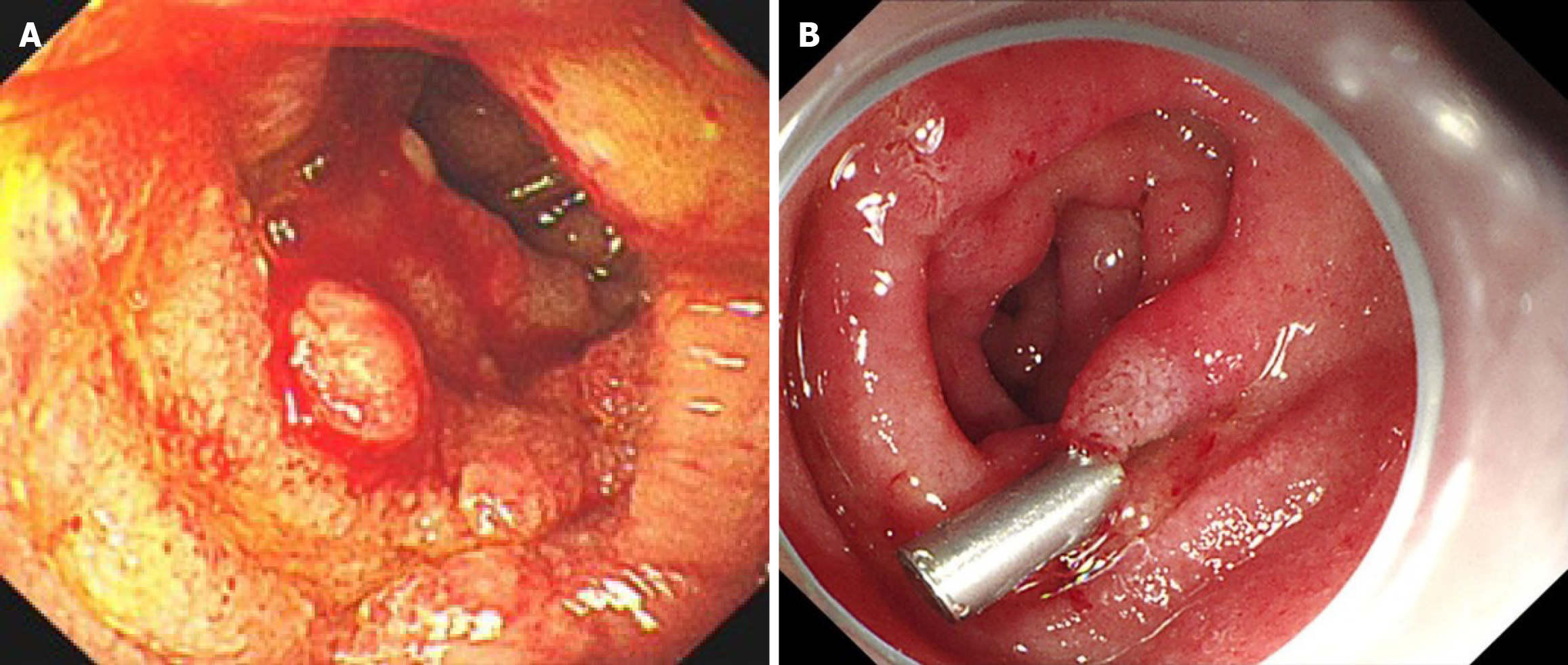
Eighteen months post-transplantation, the patient presented with abdominal cramps and diarrhea, which were subsequently accompanied by chills and fever, with a recorded maximum temperature of 102 °F. Blood cultures revealed no abnormalities. Gastroscopy identified multiple erosions, ulcers, and masses in the descending and horizontal portions of the duodenum. Biopsy pathology demonstrated fungal -like fine particles along with significant lymphocyte, plasma cell, and neutrophil infiltration in the lamina propria, findings strongly suggestive of a fungal infection. Metagenomic next-generation sequencing (mNGS) of the biopsy tissue confirmed the presence of Histoplasma capsulatum, denoted by sequence number 1677 (Figure 4A). Given the atypical presentation of the patient’s initial diagnosis, frozen liver tissue obtained during the transplantation surgery was retrieved and analyzed via mNGS. This analysis also identified Histoplasma capsulatum, with sequence number 4664 (Figure 4B). Based on these findings, a definitive diagnosis of histoplasmosis was established. Considering the patient’s prior positive response to voriconazole, antifungal therapy was resumed with voriconazole at a dosage of 200 mg every 12 hours. Blood concentrations of voriconazole and tacrolimus were regularly monitored to adjust dosages appropriately.
After 6 months of treatment, bone marrow aspiration showed no evidence of Histoplasma capsulatum (Figure 3B). Additionally, no Histoplasma capsulatum was detected in bone marrow or blood mNGS analyses. Follow-up gastroscopy revealed smooth mucosa in the duodenal bulb and the descending portion of the duodenum (Figure 5). Following this effective 6-month course of antifungal therapy, voriconazole was discontinued. As of the latest follow-up, the patient remains asymptomatic, with no recurrence of fever, abdominal pain, or diarrhea.
The presentation of histoplasmosis is intricate and nonspecific, depending on the site of involvement and the severity of infection. The most prevalent symptoms include fever (89.1%), respiratory issues (38.1%), weight loss (37.4%), and with various common pathological signs, such as enlargement of the spleen (72%), hepatomegaly (68.1%), and enlarged lymph nodes (41.2%)[6]. The liver is reportedly affected in approximately 90% of patients with disseminated histoplasmosis[7]. However, liver histoplasmosis as the primary manifestation of histoplasmosis without lung involvement is uncommon. We reviewed the relevant literature discussing hepatic histoplasmosis by searching for “Histoplasma, liver” in PubMed, nine cases of Histoplasma originating in the liver without pulmonary involvement, with manifestations ranging from mild hepatic dysfunction, granulomatous hepatitis, obstructive jaundice, to severe hepatic failure, and only two of them were immunocompetent[8,9]. Involvement of the gastrointestinal tract manifests as masses or ulcers in the gastrointestinal tract, causing pain, bleeding, perforation, or malabsorption of nutrients[1]. According to a descriptive review of 82 human immunodeficiency virus-negative patients with gastrointestinal histoplasmosis, colon was the most affected site (42.7%), and only 2 cases reported involvement of duodenum diagnosed with endoscopy. Four cases without respiratory tract involvement were immunocompromised, including hepatitis C infection, psoriatic arthritis undergoing treatment with methotrexate and infliximab, and post-liver transplant on immunosuppressive agents[10]. This is a rare and typical disseminated histoplasmosis case without pulmonary involvement in an immunocompetent individual encroaching on liver and duodenum.
Recent studies have revealed that histoplasmosis, previously believed to cause severe infections mainly in immunocompromised individuals, can also affect immunocompetent patients, sometimes resulting in a disseminated form of the disease[11]. The majority of literature reporting cases of liver histoplasmosis were in immunocompromised patients, there were four cases of disseminated histoplasmosis involved in liver in immunocompetent individuals reported in PubMed. Table 1 compares the clinical characteristics of the four cases[8,12-14]. The patient did not belong to the traditional “immunocompromised group” and lacked a definitive history of exposure. Thus, we believed there might be a possible clinical correlation between liver histoplasmosis and hepatitis B infection, though relevant studies are rare. During the pathogenesis of CHB, it is noted that there is a low expression of T-helper cells, especially Th1 subset that produce cytokines, such as interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, which affects infection control. This may be a strategy for hepatitis B to achieve immune escape by secreting hepatitis B e antigen[15]. Therefore, the possibility of specific pathogenic bacterial infections should be considered when treating patients with subacute liver failure with a history of CHB who have atypical disease.
| Ref. | Country | Sex | Age | Symptoms | Involvement | Intervention | Prognosis |
| Muhanna et al[12], 2021 | United States | Male | 62 | Abdominal pain, hepatic dysfunction, nausea, diarrhea, and anorexia | Liver | Laparoscopic cholecystectomy, ITZ for 9 months (details not reported) | Recovery |
| Gandhi et al[8], 2021 | India | Male | 60 | Fever, jaundice, abdominal pain, cough, rhinorrhea, brown-colored urine | Liver, lung, and gastrointestinal tract | AmB for 2 weeks, shifted to ITZ for 7 months, back to AmB for 2 months (details not reported) | Lost |
| Ling et al[13], 2018 | China | Female | 45 | Fever, jaundice, and hepatic dysfunction | Liver, spleen, bone marrow, and lung | ITZ for 3 weeks, shifted to AmB for a total of 9.0 g, then ITZ (200 mg bid) for 2 months | Recovery |
| Ecka et al[14], 2015 | India | Male | 62 | Fever, cough, and hepatosplenomegaly | Liver, spleen, subcarinal, and preaortic lymph node | AmB for 7 days, followed by ITZ for 2 weeks | Recovery |
Disseminated histoplasmosis can be diagnosed through various methods, such as cultures, serology, antigen detection, direct microscopy, and DNA assay for Histoplasma capsulatum. However, the atypical histopathology, low culture detection rates and long culture cycles (2 to 4 weeks, sometimes up to 8 weeks) make precise diagnosis challenging, and most patients will die after 10-14 days without prompt therapy[16]. In our patient, histiocyte phagocytosis of myco
The American Society of Transplantation guidelines recommend amphotericin B or itraconazole (ITZ) as the preferred treatment for immunocompetent patients afflicted with disseminated histoplasmosis. Furthermore, in the context of organ transplant recipients, careful adjustment of both antifungal and immunosuppressive medications is advised to minimize the potential for infection dissemination[21]. Amphotericin B is seldom used nowadays due to its pronounced nephrotoxic and ophthalmotoxic effects. The ITZ oral formulation is difficult to maintain at effective concentrations due to bioavailability limitations, and patient compliance is poor with long-range intravenous administration. A prospective study comparing voriconazole and ITZ for the prevention of fungal infections after allogeneic hematopoietic stem cell transplantation showed that voriconazole was better tolerated than ITZ when administered over a prolonged period of time (48.7% vs 33.2%, P < 0.01), whereas ITZ was associated with serious side effects, including gastrointestinal reactions such as nausea (16.6%), vomiting (15.8%), and diarrhea (10.4%)[22]. Despite the well-established hepatotoxicity of voriconazole, both experimental and clinical data indicate a generally low incidence of serious hepatic adverse events. A large meta-analysis of 24 studies demonstrated that controlling voriconazole drug concentrations at between 1.0 and 6.0 mg/L achieved efficacy while minimizing toxicity[23]. Meanwhile, commonly used calcium-modulated phosphatase inhibitors such as tacrolimus are metabolized via the cytochrome P450 3A enzyme system after transplantation, and triazoles inhibit the cytochrome P450 3A enzyme system, subsequently inhibiting tacrolimus metabolism. We treated the patient with voriconazole, which was maintained at a trough concentration of 2.0-5.0 μg/mL, while the tacrolimus dosage was reduced to 50% of the initial dosage and maintained at a concentration of 4-10 ng/mL. The infection was under control after 6 months. However, there is a paucity of literature on voriconazole for the treatment of histoplasmosis. The dosage adjustment regimen for immunosuppression after discontinuation of triazoles is equally important, and more clinical experience and research are necessary to revise the guidelines. The dose adjustment of voriconazole and the choice of individualized regimen in this case provide valuable lessons for the treatment of histoplasmosis.
Meanwhile, the course of antifungal therapy is critical for disease progression and prognosis. The American Society of Transplantation recommends that antifungal therapy should continue for at least 12 months, and indefinite treatment is advised for patients with substantial immune suppression and those who are prone to relapse[22]. We started empirical antifungal therapy with voriconazole for 3 months at the time of the initial detection of the fungus in the bone marrow smears, and stopped the medication after symptomatic control and normalization of test results. However, the patient developed a severe gastrointestinal infection 1 year later. Given the hepatotoxicity of voriconazole and compliance with the extended therapeutic regimen, empirical voriconazole treatment was reinstated for an additional 6 months. A comprehensive evaluation was performed that encompassed bone marrow, liver, and duodenal biopsies, as well as mNGS analysis. Subsequently, the patient attained complete recovery, with no recurrence of fever observed to date.
We report for the first time a case of subacute liver failure due to histoplasmosis infection successfully managed through liver transplantation in conjunction with antifungal therapy. We have drawn the following conclusions regarding the diagnostic and therapeutic process. First, histoplasmosis manifestation varies and lacks specificity, and the possibility of histoplasmosis infection should be considered when treating patients with subacute liver failure with CHB who have atypical symptoms. Second, voriconazole has shown good efficacy in the treatment of histoplasmosis; Further investigations are required to establish tailored dosing regimens and optimize strategies for therapeutic drug monitoring. Therefore, this case provides important clinical implications for the management of disseminated hepatic histoplasmosis during the perioperative phase of liver transplantation.
| 1. | Sayeed M, Benzamin M, Nahar L, Rana M, Aishy AS. Hepatic Histoplasmosis: An Update. J Clin Transl Hepatol. 2022;10:726-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Chen L, Hu D, Zhang C, Wu T, Cheng X, Hagen F, Zhu H, Deng S. Histoplasmosis: An epidemiological and clinical update in China, review and a case report. Mycology. 2024;15:101-109. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Liu X, Zhao Z, Zong Z. Precise geographical distribution and call for accurate identification of histoplasmosis cases in China. Lancet Microbe. 2024;5:100943. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Araúz AB, Papineni P. Histoplasmosis. Infect Dis Clin North Am. 2021;35:471-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Rihana NA, Kandula M, Velez A, Dahal K, O'Neill EB. Histoplasmosis presenting as granulomatous hepatitis: case report and review of the literature. Case Rep Med. 2014;2014:879535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Xiong XF, Fan LL, Kang M, Wei J, Cheng DY. Disseminated histoplasmosis: a rare clinical phenotype with difficult diagnosis. Respirol Case Rep. 2017;5:e00220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Goodwin RA, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated Histoplasmosis. Medicine. 1980;59:1-33. [RCA] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 352] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Gandhi D, Gandhi T, Wolfe A, Kichloo A, Singh J, Batts KP, Patel L. Disseminated histoplasmosis leading to end stage liver failure in immunocompetent patient: case report and review of literature. Radiol Case Rep. 2021;16:2214-2219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Numan L, Hayajneh WA, Kiwan W. Disseminated Histoplasmosis Presenting as Obstructive Jaundice. ACG Case Rep J. 2023;10:e01173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Ekeng BE, Itam-Eyo AE, Osaigbovo II, Warris A, Oladele RO, Bongomin F, Denning DW. Gastrointestinal Histoplasmosis: A Descriptive Review, 2001-2021. Life (Basel). 2023;13:689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Lamps LW, Molina CP, West AB, Haggitt RC, Scott MA. The pathologic spectrum of gastrointestinal and hepatic histoplasmosis. Am J Clin Pathol. 2000;113:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Muhanna A, Nimri FM, Almomani ZA, Al Momani L, Likhitsup A. Granulomatous Hepatitis Secondary to Histoplasmosis in an Immunocompetent Patient. Cureus. 2021;13:e17631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Ling Q, Zhu W, Lu Q, Jin T, Ding S. Disseminated histoplasmosis in an immunocompetent patient from an endemic area: A case report. Medicine (Baltimore). 2018;97:e11486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Ecka RS, Sharma M, Tomar V. Disseminated histoplasmosis in an immunocompetent haweli dweller: A diagnosis and follow-up by endoscopic ultrasound-guided fine-needle aspiration. J Cytol. 2015;32:142-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L, Fiocchi A, Vacca M, Aiolfi R, Sammicheli S, Mainetti M, Cataudella T, Raimondi A, Gonzalez-Aseguinolaza G, Protzer U, Ruggeri ZM, Chisari FV, Isogawa M, Sitia G, Iannacone M. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell. 2015;161:486-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 16. | Denning DW. Minimizing fungal disease deaths will allow the UNAIDS target of reducing annual AIDS deaths below 500 000 by 2020 to be realized. Philos Trans R Soc Lond B Biol Sci. 2016;371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Kyriakidis I, Tragiannidis A, Munchen S, Groll AH. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf. 2017;16:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Fida M, Misra A, Harring JA, Kubbara A, Theel ES. Histoplasma capsulatum Complement Fixation and Immunodiffusion Assay Sensitivity in Culture-Confirmed Cases of Histoplasmosis: a 10-Year Retrospective Review (2011 to 2020). J Clin Microbiol. 2022;60:e0105722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Zhang HC, Zhang QR, Ai JW, Cui P, Wu HL, Zhang WH, Wang T. The role of bone marrow metagenomics next-generation sequencing to differential diagnosis among visceral leishmaniasis, histoplasmosis, and talaromycosis marneffei. Int J Lab Hematol. 2020;42:e52-e54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the Leap from Research Laboratory to Clinic: Challenges and Opportunities for Next-Generation Sequencing in Infectious Disease Diagnostics. mBio. 2015;6:e01888-e01815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 21. | Cuellar-Rodriguez J, Avery RK, Lard M, Budev M, Gordon SM, Shrestha NK, van Duin D, Oethinger M, Mawhorter SD. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis. 2009;49:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Dodds-Ashley E. Management of drug and food interactions with azole antifungal agents in transplant recipients. Pharmacotherapy. 2010;30:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Luong ML, Al-Dabbagh M, Groll AH, Racil Z, Nannya Y, Mitsani D, Husain S. Utility of voriconazole therapeutic drug monitoring: a meta-analysis. J Antimicrob Chemother. 2016;71:1786-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |