TO THE EDITOR
Non-alcoholic fatty liver disease (NAFLD) is a growing global health concern, affecting 25.2% of the world’s population[1]. This public health challenge is strongly associated with the rising prevalence of obesity and metabolic syndrome[2]. Despite medical advancements, effective treatment options for NAFLD remain limited, often with undesirable side effects. Currently, no specific drug has been approved by the Food and Drug Administration for treating NAFLD[3]. Therefore, the search for new treatment options from traditional medicine is critical. A recent study published in the World Journal of Gastroenterology by Niu et al[4] titled "Fanlian Huazhuo Formula alleviates high-fat diet-induced non-alcoholic fatty liver disease by modulating autophagy and lipid synthesis signaling pathway", delves into traditional Chinese medicine (TCM) compound [Fanlian Huazhuo Formula (FLHZF)] in alleviating high-fat diet-induced NAFLD by regulating autophagy and lipid synthesis signaling pathways. FLHZF, a compound herbal preparation, contains multiple active components that regulate hepatic lipid metabolism, adipose tissue inflammation, and gut microbiota. Despite its promising therapeutic potential, evidence of its benefits in diet-induced hepatic steatosis remains limited. In this study, FLHZF was shown to alleviate lipid accumulation, oxidative stress, liver injury, and autophagy in an metabolic dysfunction-associated steatotic liver disease model by modulating key signaling pathways involved in lipid metabolism[5]. However, the multifaceted and multitarget nature of Chinese medicine impedes research progress. This study evaluated the potential of TCM for the treatment of NAFLD and its research significance from various perspectives.
Study overview and discussion
NAFLD is a complex metabolic disorder strongly associated with obesity, diabetes, dyslipidemia, and cardiovascular disease[6]. Current treatments primarily involves lifestyle interventions, including low-calorie diets (particularly the Mediterranean diet) and regular physical activity. Weight loss of 5%-10% has been shown to substantially improve hepatic steatosis and inflammation[7]. However, no pharmacological therapies are currently approved specifically for NAFLD, and existing options have demonstrated limited efficacy and safety. For example, while pioglitazone can reduce hepatic steatosis and inflammation, it may induce weight gain[8]. Similarly, bile acid receptor agonists offer benefits in alleviating liver fibrosis but can lead to elevated cholesterol levels and pruritus[9]. Vitamin E is recommended for patients with non-diabetic non-alcoholic steatohepatitis, but prolonged use raises concerns regarding bleeding risk[10]. Emerging treatments, such as probiotics and vitamin D, show promise in improving gut microbiota and liver function, but strong clinical evidence remains insufficient[11,12].
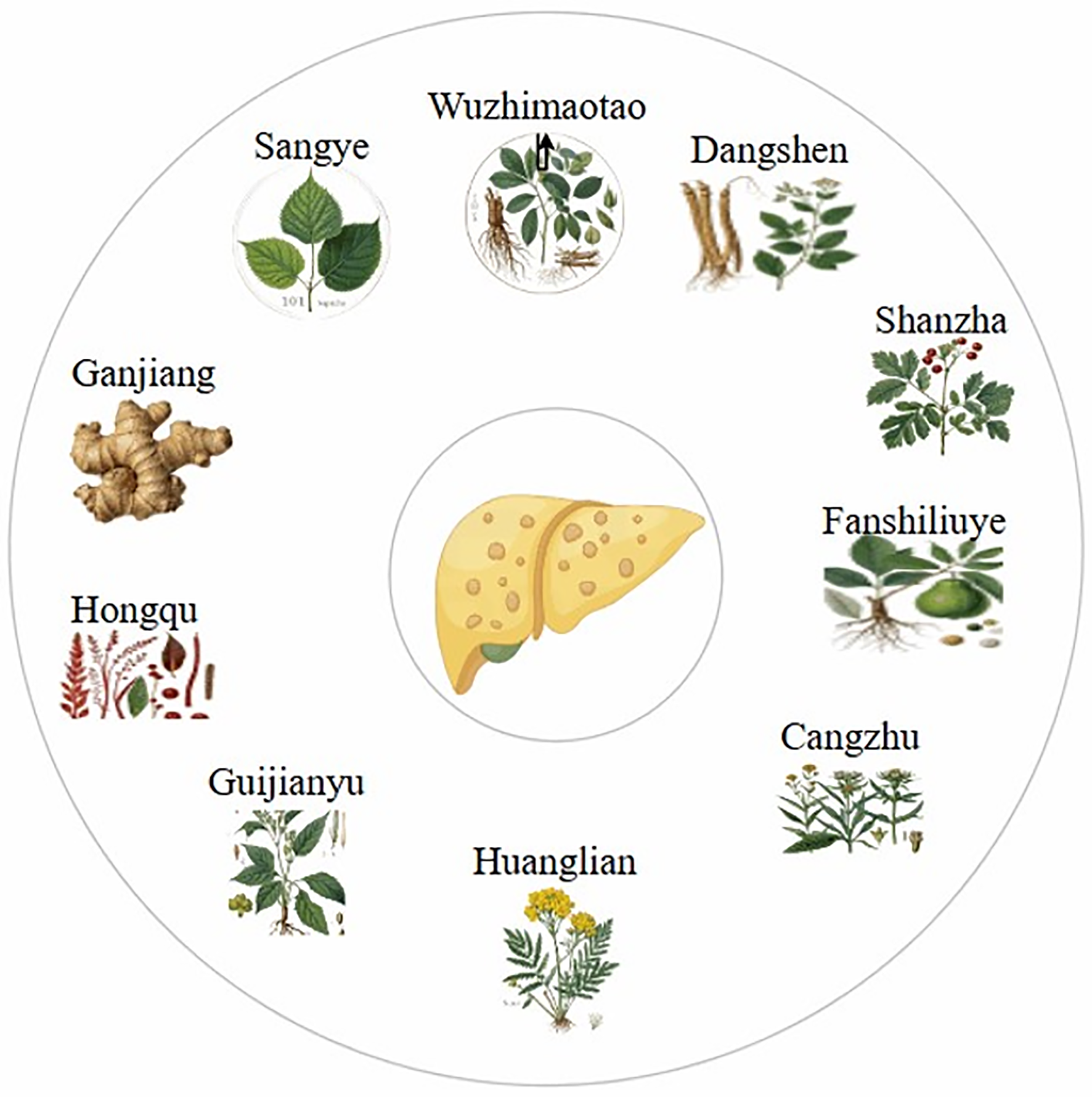
TCM, recognized for its efficacy and minimal side effects, has been widely used for the prevention and treatment of NAFLD[13]. The herbal compound FLHZF, comprising 10 herbal components (Dangshen, Shanzha, Fanshiliuye, Cangzhu, Huanglian, Guijinayu, Hongqu, Ganjiang, Sangye, and Wuzhimaotao), has demonstrated effectiveness in animal and cellular studies (Figure 1)[14]. FLHZF mitigates NAFLD by regulating lipid metabolism, reducing oxidative stress, and activating autophagy. It also improves gut microbiota composition, lowers endotoxin levels, and alleviates systemic inflammation. However, randomized clinical trials evaluating FLHZF for the treatment of NAFLD have not yet been conducted, necessitating further research to elucidate its mechanism of action and clinical utility. Silymarin, another promising TCM compound, has shown therapeutic potential in patients with mild to moderate liver damage. Its antioxidant, anti-inflammatory, antifibrotic, and insulin-sensitizing effects, have been validated in multiple clinical studies, highlighting its utility in managing NAFLD.
Figure 1 Treating non-alcoholic fatty liver disease with Fanlian Huazhuo Formula.
Recent studies underscore the significant roles of TCM compounds[15,16], natural products[17], and individual herbal components[18] in the prevention and mitigation of NAFLD progression. TCM’s holistic and syndrome differentiation-based approaches focus on liver protection through mechanisms such as oxidative stress reduction, lipid metabolism regulation, anti-inflammatory and anti-fibrotic effects, and gut microbiota modulation. These distinctive advantages position TCM as a promising strategy to address the complexities of NAFLD. Future research should prioritize identifying shared mechanisms underlying TCM-based therapies to further their clinical application and scientific advancement.
Strengths and limitations
TCM offers advantages in treating NAFLD due to its holistic and dialectical approach. However, the "multiple components, multiple targets, and multiple pathways" characteristics of Chinese herbal medicine, especially herbal compounds, pose challenges for research. This targeted pathway-level comprehensive regulation of herbal medicines makes them more competitive than other chemicals or active ingredients. Niu et al[4] systematically investigated the therapeutic effects and mechanisms of FLHZF in NAFLD using in vivo and in vitro experiments. However, the study has some limitations. First, as a herbal compound, FLHZF's pharmacological effects of FLHZF involve multiple targets and pathways. Future research should analyze FLHZF components in the bloodstream to better understand their metabolic processes in vivo. In addition, network pharmacology could be used to further explore the targets of FLHZF in the treatment of NAFLD. Second, in the in vitro cell experiments conducted by Niu et al[4] freeze-dried FLHZF powder was used for intervention. A more accurate approach would involve using FLHZF-containing serum in these experiments to simulate the absorption of active components in the bloodstream after herbal medicine metabolism. This adjustment would more accurately reflect the actual efficacy and pharmacological effects of the formula in vivo. Lastly, while the components of FLHZF are traditional Chinese medicinal herbs, rigorous clinical safety testing is essential to improve the objectivity and reliability of the findings. This represents a critical direction for future studies.
Future research directions
NAFLD is a complex metabolic disorder characterized by liver steatosis, inflammation, and fibrosis. Currently, no specific drugs are available for NAFLD treatment. TCM, with its holistic approach and multicomponent herbal formulations, offers a promising alternative. However, research on the mechanism of action of Chinese herbal medicines for NAFLD has limitations. First, these mechanisms are not fully understood. Second, there are a few registered clinical trials of herbal compounds. While some have shown efficacy in animal models, the long-term efficacy of TCM is difficult to assess due to limited clinical sample sizes and lack of long-term follow-up studies. Future development of Chinese herbal medicine for NAFLD requires a comprehensively understanding of the activity and mechanism of action of herbal formulations. With the innovative development of network pharmacology, bioinformatics, and computational technology, TCM and computational systems have become increasingly integrated. Combining network pharmacology with systems biology, omics, bioinformatics, and other disciplines allows for a more sophisticated investigation of Chinese herbal medicine based on the "disease-gene-target-drug" interaction network. The application of these omics analysis methods aligns with the holistic concept of regulating relevant targets and signaling pathways to achieve a comprehensive therapeutic effect on NAFLD. This approach not only embodies the innovative concept of "dialectical treatment" in TCM, but also expands the application scope of Chinese herbal medicine, promotes its modernization, and aligns with the future development trends of TCM. The active ingredients, targets, mechanism of action, compatibility rules, and pharmacological basis of TCM for treating NAFLD are described more clearly, providing a systematic perspective for future research on the mechanism of action of Chinese herbal medicines.
CONCLUSION
TCM has unique advantages and potential for treating NAFLD. Its mechanisms of action are multifaceted, including anti-inflammatory, antioxidant, lipid-lowering, and liver-protective activities. Modern techniques, like network pharmacology, omics, and bioinformatics, can enhance our understanding of the mechanisms of action and active ingredients of Chinese herbal medicines, promoting their modernization. This not only helps improve the efficacy of TCM in the treatment of NAFLD but also paves the way for the development of Chinese herbal medicine. In the future, as research on TCM continues to deepen and innovate, identifying commonalities in Chinese herbal medicine treatments for NAFLD, enriching the scientific content of TCM theory, and exploring the treatment of NAFLD using TCM will lead to greater efficacy in the treatment of metabolic disorders such as NAFLD.