Published online Feb 28, 2025. doi: 10.3748/wjg.v31.i8.101357
Revised: December 29, 2024
Accepted: January 6, 2025
Published online: February 28, 2025
Processing time: 133 Days and 15.2 Hours
The recent study published in the World Journal of Gastroenterology examines the interplay among the neuroendocrine axis, gut microbiota, inflammatory markers, and gastrointestinal symptoms in irritable bowel syndrome (IBS). By integrating all these factors into a single study, this approach reflects the modern concept of functional gastrointestinal disorders as disorders of the gut-brain interaction to be approached in a multiparametric manner, also incorporating non-gastroenterological elements and extending evaluations to parameters related to the neuroendocrine axis. This invited letter to the editor summarizes the main results of the aforementioned study and highlights its multiparametric approach, including variables not strictly gastroenterological, in the study of IBS, and discusses its strengths and limitations.
Core Tip: This letter highlights the complex interplay among the neuroendocrine axis, gut microbiota, and inflammatory response in irritable bowel syndrome (IBS). By adopting a multiparametric approach, the findings proposed by the commented article suggest that IBS involves both gastrointestinal and neuroendocrine factors, supporting the biopsychosocial model for understanding and managing functional gastrointestinal disorders.
- Citation: Pellegrino R, Gravina AG. Irritable bowel syndrome remains a complex disorder of gut-brain interaction: Too many actors on stage. World J Gastroenterol 2025; 31(8): 101357
- URL: https://www.wjgnet.com/1007-9327/full/v31/i8/101357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i8.101357
We read with interest the recent study published in the World Journal of Gastroenterology by Zhang et al[1] entitled, “Correlation between the neuroendocrine axis, microbial species, inflammatory response, and gastrointestinal symptoms in irritable bowel syndrome”, in which the authors compared established elements of the postulated pathogenic hypotheses for irritable bowel syndrome (IBS), including factors related to the neuroendocrine axis, gut microbiota, and pro-inflammatory cytokines.
The authors recruited, over 1 year, 80 patients with IBS [most of whom had IBS with diarrhea (IBS-D)] and an equal number of healthy controls, ensuring sample homogeneity in terms of sex, age, body mass index, and educational level. They collected neuroendocrine variables [cortisol, adrenocorticotropic hormone (ACTH), serotonin (5-HT)], serum inflammatory markers [interleukin 6 (IL-6), IL-8, and IL-10], clinical data (gastrointestinal symptoms assessed using the Bristol stool form scale and the ROME III diagnostic criteria for functional gastrointestinal disorders), and gut microbiota characteristics (using the 16S rRNA technique). The results demonstrated higher levels of ACTH, cortisol, and serotonin (5-HT) in patients with IBS compared to controls, with no intragroup differences among IBS phenotypes [IBS-D, IBS with constipation (IBS-C), and a combination of IBS-D and IBS-C]. Regarding gut microbiota, Bacteroides were more prevalent in the IBS group than in controls, while Bifidobacteria, Lactobacillus, and Clostridium, showed opposite trends.
Pro-inflammatory cytokine levels (IL-6, IL-8) were higher, and one anti-inflammatory cytokine (IL-10) assessed was lower in the IBS group than in healthy controls. An intriguing finding was the greater increase in ACTH (and cortisol) levels among diarrhea-predominant patients compared to constipation-predominant patients at all measurement points (8:00 am, 4:00 pm, and 12:00 am).
There is nothing new under the sun regarding each of these elements individually. Still, comparing all these factors within the same research setting was interesting. This multidimensional approach will likely become increasingly necessary in light of the well-known and historical shift that has legitimized IBS (C1 class according to ROME IV criteria) as an essential part of a highly diverse group of functional gastrointestinal disorders, with its specific diagnostic criteria[2]. This transition moves away from viewing IBS merely as a diagnosis of exclusion or a fallback for clinicians when organic gastrointestinal disorders have been ruled out[3]. As a result, IBS has emerged as a complex product of a biopsychosocial mechanism in which genetic, epigenetic, and environmental factors interact with psychosocial elements
Although it is beyond the scope of this letter to review the pathophysiological evidence of IBS, some preliminary summary considerations can be made to approach the topic. Several factors are, therefore, involved in the pathophy
It is well known from genome-wide studies that patients with IBS are associated with specific variants on chromosome 9 at the 9q31.2 Locus (single nucleotide polymorphism rs10512344), which has been primarily linked to the regulation of cellular ion transport membrane, mutations in the sucrase-isomaltase gene, as well as autonomic dysfunction[5-7]. Staying within the realm of ion channel regulation, additional genetic variants associated with IBS involve the sodium voltage-gated channel alpha subunit 5A gene, which encodes sodium ion channels explicitly expressed in the gastrointestinal tract, affecting both smooth muscle cells and interstitial cells of Cajal[8]. In addition to these findings, other genes related to neurotransmission have been implicated in IBS, such as polymorphisms in genes involved in 5-HT biosynthesis [such as tryptophan hydroxylase (TPH), TPH in isoforms 1 and 2] and genes associated with its reuptake (5-HT reuptake transporter)[9].
It also plays a role, as epigenetic modifications that contribute to dysregulation of the gut-brain axis have been described in IBS. These modifications primarily involve microRNAs that regulate mediators of visceral pain sensitivity (such as 5-HT3) and modulate histone acetylation activity on target genes, both positively and negatively[10]. In particular, it has been observed that in patients with IBS, there are modifications in DNA methylation in key genes primarily involved in oxidative stress (such as glutathione-S-transferases Mu 5) and also implicated in the regulation of the adrenal axis, particularly the corticotropin-releasing factor gene[9].
Gut microbiota bidirectionally contributes to pathogenesis and can also be influenced by IBS. It remains challenging, even today, to define an utterly healthy microbiota for comparison with that of patients with IBS. However, it is known that IBS patients can benefit from the elimination of gut microbiota fermentable food components, such as fermentable oligosaccharides, diosaccharides, and monosaccharides and polyols (FODMAPs)[11,12]. Numerous bacterial species have been described as altered in IBS patients and differing levels of short-chain fatty acids[11]. It is also known that structural and functional alterations in the gut epithelial barrier in IBS promote low-grade inflammatory activity at this level[13]. This leads to increased intestinal permeability, allowing greater passage of both microbiota elements and their metabolites outside the intestinal lumen, further stimulating the low-grade inflammatory burden[13]. These changes are also associated with variations in the expression of pro-inflammatory cytokines and intestinal toll-like receptors[13].
Environmental risk factors linked to IBS are indeed numerous. In addition to the already mentioned diet relationship between IBS and FODMAPs, it has also been reported that a diet rich in sugars and fats (of the Western type) increases the risk of IBS[14]. It is also well established that IBS can be post-infectious following infection by various pathogens, including but not limited to Campylobacter jejuni, Escherichia coli, Salmonella enterica serovar Typhimurium, Clostridioides difficile, Vibrio cholerae, and Giardia lamblia[15]. Other behaviors, including cigarette smoking and alcohol consumption, have been associated with IBS[16-18].
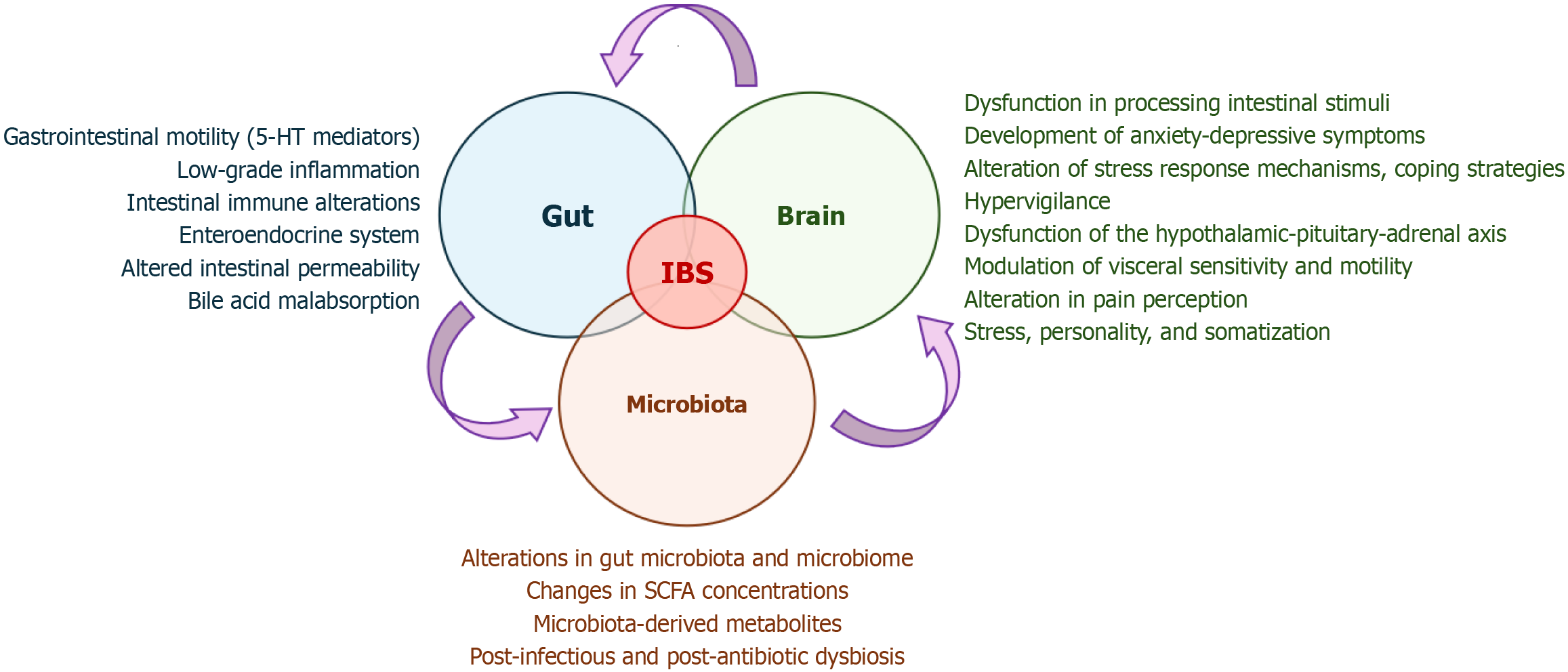
However, all these aspects and the neuropsychological factors contribute to the pathophysiological model of the microbiota-gut-brain axis (Figure 1)[19]. Psychological comorbidities in patients with IBS are highly prevalent, including stress and anxiety-depressive syndromes (which reach a prevalence of over 30% in these patients), in a bidirectional relationship of reciprocal influence[15]. A bidirectional interaction thus occurs between the central nervous system and the enteric nervous system, where the central nervous system modulates visceral sensitivity and motility, influencing the symptoms in IBS patients[20]. Conversely, the intestinal microenvironment, both in its neuroimmunological components and microbiome and microbiota, sends signals to the central nervous system[20].
In light of all these considerations, studies focusing on IBS should likely adopt this perspective, considering not only parameters related to specific gastrointestinal categorization (such as symptoms and endoscopic findings) but also examining non-gut-specific variables of psychological and neuroendocrine origin.
For this reason, ROME IV has laid the groundwork for a revolution, likely to continue with ROME V, wherein functional gastrointestinal disorders are defined as "disorders of gut-brain interaction"[21]. This approach integrates most functional disorders with a psychological perspective, particularly cognitive-behavioral approaches, which run parallel to gastroenterological strategies to manage functional symptoms directly[21].
IBS is, to date, an epidemiologically significant condition, as large-scale population studies estimate that over 40% of the sample population experience a functional gastrointestinal disorder[22].
It is not surprising that ROME IV has incorporated functional gastrointestinal disorders, such as those in category D (i.e. centrally mediated disorders of gastrointestinal pain), whose diagnostic criteria include minimal gastroenterological aspects but a significant focus on central nervous system involvement[23]. Their therapeutic algorithms predominantly involve psychological and psychiatric interventions, with no gut-specific treatments[23]. This underscores the need to move beyond solely gastroenterological parameters, such as bowel movement frequency, abdominal pain, and stool consistency, as the sole determinants of the patient's clinical picture.
It is easy, however, to speak of a functional disorder while overlooking any consideration of potential "organic" factors in IBS, and the authors reopen this chapter with some of their findings. Their data show that patients with IBS, compared to controls, exhibited a higher pro-inflammatory burden (evident in increased serum levels of IL-6 and IL-8) and a reduced anti-inflammatory response (reflected in lower serum levels of IL-10).
This finding also highlights and echoes the concept of mucosal inflammation, which can coexist even in a functional disorder like IBS. This phenomenon has already been reported and extensively debated[24-26].
Notably, one of the most established markers of intestinal inflammation, fecal calprotectin, is a reliable indicator for distinguishing suspected inflammatory bowel disease from IBS[27]. However, patients with IBS may still present with elevated levels of this marker[28]. Conversely, in patients with inflammatory bowel disease, an IBS overlap can commonly occur[29-31], suggesting a bidirectional relationship. On the one hand, IBS may find more fertile ground in a condition that is highly debilitating and disrupts psychosocial balance[32,33]; on the other hand, IBS may develop within an already altered inflammatory environment, contributing to its microinflammatory pathogenic component[26].
It is, therefore, not surprising that mucosal inflammation (i.e. low-grade inflammation), although not reaching the characteristic and paradigmatic levels seen in its "organic disease" counterpart, inflammatory bowel disease, can also exist in a functional disorder like IBS as a potential pathogenic factor. However, it may not be sufficiently pronounced to warrant being a primary therapeutic target, as current international guidelines do not recommend robust immunomodulatory therapies as a treatment strategy for IBS[34-38].
In the authors' data, the pro-inflammatory markers also correlated with the variables they employed for analyzing the neuroendocrine axis (i.e. ACTH, cortisol, and 5-HT levels).
Indeed, the melanocortin system, once a neglected area of gastrointestinal research[39], is now emerging as a promising avenue due to its potential influence on pro-inflammatory and anti-inflammatory processes. Recently, we published our data in the World Journal of Gastroenterology, demonstrating an increased expression of melanocortin receptors 3 and 5, which have an affinity for ACTH, in the colons of patients with ulcerative colitis and Crohn's disease[40]. Moreover, one of our literature reviews revealed melanocortins have an inextricable role in regulating inflammatory processes throughout the body[41].
In the authors' data, ACTH, cortisol, and 5-HT levels were significantly higher in patients with IBS than the controls. These findings had previously been reported[42,43] to highlight that patients with IBS, due to an altered response to corticotropin-releasing hormone, exhibit a reduced capacity for resilience and stress response, which aligns well with the biopsychosocial model already mentioned.
A more detailed study of the hypothalamic-pituitary-adrenal axis in the context of IBS could help elucidate why patients exhibit both the genesis of stress and a diminished ability to manage stress—an essential aspect of the physiological functioning of this axis[44]. Park et al[45], in a comparative study between IBS patients and healthy controls, demonstrated that IBS patients show reduced resilience, a finding that correlates with more severe gastrointestinal symptoms. Furthermore, stress-related psychiatric disorders, such as post-traumatic stress disorder, represent a significant risk factor for IBS, with an estimated odds ratio as high as 2.80 in recent meta-analyses[46]. Our research group has recently identified that academic stress can also be associated with a higher prevalence of IBS (according to ROME IV criteria) in a sample of medical and nurse students, alongside elevated levels of anxiety[47].
The serotonergic system is another major pathogenic factor studied in patients with IBS[48,49], and therapeutic interventions have been developed targeting this system, specifically through 5-HT3 receptor antagonism[50] and 5-HT4 receptor[51] agonism.
In support of the notion that these elements are not merely speculative, the authors provided data showing that circadian levels of ACTH and cortisol were more pronounced in patients with active diarrheal symptoms.
As is well known, the "higher" neuroendocrine and brain components can influence the gut microbiota and intestinal permeability[52]. Thus, it is unsurprising that the authors' data revealed more severe diarrheal symptoms in cases where these components are more compromised.
However, this study has the limitation of reduced generalizability, as it not only focuses on a specific population (i.e. Chinese) but also relies on patient inclusion based on national diagnostic criteria, which may not necessarily align with international standards. ROME IV has produced a comprehensive questionnaire rich in questions designed to provide diagnostic insights based on the available criteria for functional gastrointestinal disorders[53]. This tool helps navigate the numerous functional clinical entities with which IBS can easily be confused.
We believe standardizing diagnostic criteria for IBS is essential, considering that even within the criteria developed by the same source (ROME), there is significant variation between ROME III and ROME IV. The latter tends to "underestimate" IBS compared to the former due to its more stringent requirements[54].
Nonetheless, the authors excluded patients taking neuromodulators, strengthening the findings concerning IBS overall. However, given the study's small sample size, including an additional group of IBS patients treated with neuromodulators would have been interesting. This could have provided insights into whether these medications might have influenced the parameters observed, either positively or negatively. This is particularly relevant given that ROME IV has positioned neuromodulators as a critical element in managing functional gastrointestinal disorders, establishing a dedicated consensus[55] for their use in this context. Additionally, it could have been helpful to assess IBS-related symptoms using questionnaires such as the IBS symptom severity scale[56]. This would have provided a more standardized evaluation of symptom severity and enhanced the clinical relevance of the findings[57].
This all points to the need to pursue what, in our view, should be the future priorities of IBS research. It is essential to clarify better and dissect the brain-gut-microbiota interactions to identify modifiable and pharmacologically targetable elements and biomarkers. This can be achieved by understanding the neuroimmunological and neuroendocrine mechanisms underlying these interaction phenomena. In light of the existing data, it is essential to clarify further which genetic (and ideally epigenetic) variations and polymorphisms are most strongly associated with IBS, mainly those that determine the phenotypic variations among the different forms of IBS.
In conclusion, this study by Zhang et al[1] confirms that IBS is a complex puzzle of interrelated factors that shape and influence each other to produce gastrointestinal symptoms. It provides an impetus for conducting similar multiparametric studies to elucidate these intricate interactions further.
The need for this greater understanding reflects the necessity to identify effective therapeutic agents capable of fully engaging with the pathogenesis of the disease. It is true that in the non-functional counterpart of IBS, namely inflammatory bowel disease, there has been repeated recognition of the need to break through the therapeutic ceiling[58]. Despite increasing available therapies, many patients fail to achieve remission[58]. This is equally true for IBS, where[59,60], despite the proposed treatments, we still do not have a therapeutic agent/procedure[61-65] capable of dramatically controlling the pathophysiology of IBS in all patients.
| 1. | Zhang X, Jin WW, Wang HG. Correlation between the neuroendocrine axis, microbial species, inflammatory response, and gastrointestinal symptoms in irritable bowel syndrome. World J Gastroenterol. 2024;30:3985-3995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (2)] |
| 2. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1898] [Article Influence: 210.9] [Reference Citation Analysis (3)] |
| 3. | Drossman DA. The functional gastrointestinal disorders and the Rome II process. Gut. 1999;45 Suppl 2:II1-II5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 169] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Oświęcimska J, Szymlak A, Roczniak W, Girczys-Połedniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Bonfiglio F, Zheng T, Garcia-Etxebarria K, Hadizadeh F, Bujanda L, Bresso F, Agreus L, Andreasson A, Dlugosz A, Lindberg G, Schmidt PT, Karling P, Ohlsson B, Simren M, Walter S, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Portincasa P, Bellini M, Barbara G, Latiano A, Hübenthal M, Thijs V, Netea MG, Jonkers D, Chang L, Mayer EA, Wouters MM, Boeckxstaens G, Camilleri M, Franke A, Zhernakova A, D'Amato M. Female-Specific Association Between Variants on Chromosome 9 and Self-Reported Diagnosis of Irritable Bowel Syndrome. Gastroenterology. 2018;155:168-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Garcia-Etxebarria K, Zheng T, Bonfiglio F, Bujanda L, Dlugosz A, Lindberg G, Schmidt PT, Karling P, Ohlsson B, Simren M, Walter S, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Portincasa P, Bellini M, Barbara G, Jonkers D, Eswaran S, Chey WD, Kashyap P, Chang L, Mayer EA, Wouters MM, Boeckxstaens G, Camilleri M, Franke A, D'Amato M. Increased Prevalence of Rare Sucrase-isomaltase Pathogenic Variants in Irritable Bowel Syndrome Patients. Clin Gastroenterol Hepatol. 2018;16:1673-1676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Henström M, Diekmann L, Bonfiglio F, Hadizadeh F, Kuech EM, von Köckritz-Blickwede M, Thingholm LB, Zheng T, Assadi G, Dierks C, Heine M, Philipp U, Distl O, Money ME, Belheouane M, Heinsen FA, Rafter J, Nardone G, Cuomo R, Usai-Satta P, Galeazzi F, Neri M, Walter S, Simrén M, Karling P, Ohlsson B, Schmidt PT, Lindberg G, Dlugosz A, Agreus L, Andreasson A, Mayer E, Baines JF, Engstrand L, Portincasa P, Bellini M, Stanghellini V, Barbara G, Chang L, Camilleri M, Franke A, Naim HY, D'Amato M. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel syndrome. Gut. 2018;67:263-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 8. | Verstraelen TE, Ter Bekke RM, Volders PG, Masclee AA, Kruimel JW. The role of the SCN5A-encoded channelopathy in irritable bowel syndrome and other gastrointestinal disorders. Neurogastroenterol Motil. 2015;27:906-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Mahurkar-Joshi S, Chang L. Epigenetic Mechanisms in Irritable Bowel Syndrome. Front Psychiatry. 2020;11:805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Dothel G, Barbaro MR, Di Vito A, Ravegnini G, Gorini F, Monesmith S, Coschina E, Benuzzi E, Fuschi D, Palombo M, Bonomini F, Morroni F, Hrelia P, Barbara G, Angelini S. New insights into irritable bowel syndrome pathophysiological mechanisms: contribution of epigenetics. J Gastroenterol. 2023;58:605-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396:1675-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 452] [Article Influence: 90.4] [Reference Citation Analysis (2)] |
| 12. | Altobelli E, Del Negro V, Angeletti PM, Latella G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients. 2017;9:940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 13. | Hillestad EMR, van der Meeren A, Nagaraja BH, Bjørsvik BR, Haleem N, Benitez-Paez A, Sanz Y, Hausken T, Lied GA, Lundervold A, Berentsen B. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J Gastroenterol. 2022;28:412-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (4)] |
| 14. | Buscail C, Sabate JM, Bouchoucha M, Kesse-Guyot E, Hercberg S, Benamouzig R, Julia C. Western Dietary Pattern Is Associated with Irritable Bowel Syndrome in the French NutriNet Cohort. Nutrients. 2017;9:986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 16. | Zvolensky MJ, Smit T, Dragoi I, Tamminana R, Bakhshaie J, Ditre JW, Redmond BY, Lackner J. Irritable Bowel Syndrome (IBS) and Smoking: An Evaluation of IBS symptom severity and anxiety sensitivity among adults in the United States. Addict Behav. 2025;160:108187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 17. | Ho FF, Sun H, Zheng H, Wong DCN, Gao YY, Mao C, Cheung YT, Lam CS, Wang MH, Wu IX, Wu JCY, Chung VCH. Association of healthy lifestyle behaviours with incident irritable bowel syndrome: a large population-based prospective cohort study. Gut. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Yuan S, Chen J, Ruan X, Sun Y, Zhang K, Wang X, Li X, Gill D, Burgess S, Giovannucci E, Larsson SC. Smoking, alcohol consumption, and 24 gastrointestinal diseases: Mendelian randomization analysis. Elife. 2023;12:e84051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 114] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 19. | Moser G, Fournier C, Peter J. Intestinal microbiome-gut-brain axis and irritable bowel syndrome. Wien Med Wochenschr. 2018;168:62-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Chen M, Ruan G, Chen L, Ying S, Li G, Xu F, Xiao Z, Tian Y, Lv L, Ping Y, Cheng Y, Wei Y. Neurotransmitter and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front Endocrinol (Lausanne). 2022;13:817100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 21. | Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. 2017;23:151-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 22. | Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EMM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1196] [Article Influence: 299.0] [Reference Citation Analysis (0)] |
| 23. | Keefer L, Drossman DA, Guthrie E, Simrén M, Tillisch K, Olden K, Whorwell PJ. Centrally Mediated Disorders of Gastrointestinal Pain. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 373] [Article Influence: 41.4] [Reference Citation Analysis (1)] |
| 25. | De Silva AP, Nandasiri SD, Hewavisenthi J, Manamperi A, Ariyasinghe MP, Dassanayake AS, Jewell DP, de Silva HJ. Subclinical mucosal inflammation in diarrhea-predominant irritable bowel syndrome (IBS) in a tropical setting. Scand J Gastroenterol. 2012;47:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS). J Inflamm Res. 2018;11:345-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (2)] |
| 27. | Dajti E, Frazzoni L, Iascone V, Secco M, Vestito A, Fuccio L, Eusebi LH, Fusaroli P, Rizzello F, Calabrese C, Gionchetti P, Bazzoli F, Zagari RM. Systematic review with meta-analysis: Diagnostic performance of faecal calprotectin in distinguishing inflammatory bowel disease from irritable bowel syndrome in adults. Aliment Pharmacol Ther. 2023;58:1120-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Caviglia GP, Ribaldone DG, Rosso C, Saracco GM, Astegiano M, Pellicano R. Fecal calprotectin: beyond intestinal organic diseases. Panminerva Med. 2018;60:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Lim J, Rezaie A. Irritable Bowel Syndrome-Like Symptoms in Quiescent Inflammatory Bowel Disease: A Practical Approach to Diagnosis and Treatment of Organic Causes. Dig Dis Sci. 2023;68:4081-4097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Baillie S, Norton C, Saxena S, Pollok R. Chronic abdominal pain in inflammatory bowel disease: a practical guide. Frontline Gastroenterol. 2024;15:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 31. | Soheilipour M, Chermahini TG, Tamizifar B, Kassaian N, Khorasani MR, Adibi P. Relative Frequency of Gastrointestinal Functional Disorders in Patients with Inflammatory Bowel Disease Based on Rome IV: A Case-Control Study. Adv Biomed Res. 2024;13:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Ananthakrishnan AN, Kaplan GG, Bernstein CN, Burke KE, Lochhead PJ, Sasson AN, Agrawal M, Tiong JHT, Steinberg J, Kruis W, Steinwurz F, Ahuja V, Ng SC, Rubin DT, Colombel JF, Gearry R; International Organization for Study of Inflammatory Bowel Diseases. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol. 2022;7:666-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 33. | Tavakoli P, Vollmer-Conna U, Hadzi-Pavlovic D, Grimm MC. A Review of Inflammatory Bowel Disease: A Model of Microbial, Immune and Neuropsychological Integration. Public Health Rev. 2021;42:1603990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 34. | Di Nardo G, Barbara G, Borrelli O, Cremon C, Giorgio V, Greco L, La Pietra M, Marasco G, Pensabene L, Piccirillo M, Romano C, Salvatore S, Saviano M, Stanghellini V, Strisciuglio C, Tambucci R, Turco R, Zenzeri L, Staiano A. Italian guidelines for the management of irritable bowel syndrome in children and adolescents : Joint Consensus from the Italian Societies of: Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP), Pediatrics (SIP), Gastroenterology and Endoscopy (SIGE) and Neurogastroenterology and Motility (SINGEM). Ital J Pediatr. 2024;50:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 35. | McKenzie YA, Bowyer RK, Leach H, Gulia P, Horobin J, O'Sullivan NA, Pettitt C, Reeves LB, Seamark L, Williams M, Thompson J, Lomer MC; (IBS Dietetic Guideline Review Group on behalf of Gastroenterology Specialist Group of the British Dietetic Association). British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). J Hum Nutr Diet. 2016;29:549-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 237] [Article Influence: 26.3] [Reference Citation Analysis (3)] |
| 36. | Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116:17-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 469] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 37. | Savarino E, Zingone F, Barberio B, Marasco G, Akyuz F, Akpinar H, Barboi O, Bodini G, Bor S, Chiarioni G, Cristian G, Corsetti M, Di Sabatino A, Dimitriu AM, Drug V, Dumitrascu DL, Ford AC, Hauser G, Nakov R, Patel N, Pohl D, Sfarti C, Serra J, Simrén M, Suciu A, Tack J, Toruner M, Walters J, Cremon C, Barbara G. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United European Gastroenterol J. 2022;10:556-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 38. | Barbara G, Cremon C, Bellini M, Corsetti M, Di Nardo G, Falangone F, Fuccio L, Galeazzi F, Iovino P, Sarnelli G, Savarino EV, Stanghellini V, Staiano A, Stasi C, Tosetti C, Turco R, Ubaldi E, Zagari RM, Zenzeri L, Marasco G. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig Liver Dis. 2023;55:187-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 39. | Montero-Melendez T. ACTH: The forgotten therapy. Semin Immunol. 2015;27:216-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 40. | Gravina AG, Panarese I, Trotta MC, D'Amico M, Pellegrino R, Ferraraccio F, Galdiero M, Alfano R, Grieco P, Federico A. Melanocortin 3,5 receptors immunohistochemical expression in colonic mucosa of inflammatory bowel disease patients: A matter of disease activity? World J Gastroenterol. 2024;30:1132-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Reference Citation Analysis (0)] |
| 41. | Gravina AG, Pellegrino R, Durante T, Palladino G, Imperio G, D'Amico G, Trotta MC, Dallio M, Romeo M, D'Amico M, Federico A. The Melanocortin System in Inflammatory Bowel Diseases: Insights into Its Mechanisms and Therapeutic Potentials. Cells. 2023;12:1889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Reference Citation Analysis (0)] |
| 42. | Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42 Suppl 17:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Kano M, Muratsubaki T, Van Oudenhove L, Morishita J, Yoshizawa M, Kohno K, Yagihashi M, Tanaka Y, Mugikura S, Dupont P, Ly HG, Takase K, Kanazawa M, Fukudo S. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7:12425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 45. | Park SH, Naliboff BD, Shih W, Presson AP, Videlock EJ, Ju T, Kilpatrick L, Gupta A, Mayer EA, Chang L. Resilience is decreased in irritable bowel syndrome and associated with symptoms and cortisol response. Neurogastroenterol Motil. 2018;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Ng QX, Soh AYS, Loke W, Venkatanarayanan N, Lim DY, Yeo WS. Systematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol. 2019;34:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 47. | Gravina AG, Pellegrino R, Romeo M, Palladino G, Cipullo M, Iadanza G, Olivieri S, Zagaria G, Mazzarella C, Durante T, Federico A. The Burden of Irritable Bowel Syndrome in Medical and Nurse Italian University Student Population: The VANVITELLI-IBS Survey. Rev Recent Clin Trials. 2023;18:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Binienda A, Storr M, Fichna J, Salaga M. Efficacy and Safety of Serotonin Receptor Ligands in the Treatment of Irritable Bowel Syndrome: A Review. Curr Drug Targets. 2018;19:1774-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Gros M, Gros B, Mesonero JE, Latorre E. Neurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Management. J Clin Med. 2021;10:3429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 50. | Gunn D, Topan R, Fried R, Holloway I, Brindle R, Hartley S, Barnard L, Corsetti M, Scott SM, Farmer A, Akbar A, Eugenicos M, Trudgill N, Kapur K, McLaughlin J, Sanders DS, Ramadas A, Whorwell P, Houghton L, Dinning PG, Aziz Q, Ford AC, Farrin A, Spiller R. Ondansetron for irritable bowel syndrome with diarrhoea: randomised controlled trial. Southampton (UK): National Institute for Health and Care Research; 2023. [PubMed] [DOI] [Full Text] |
| 51. | Madia VN, Messore A, Saccoliti F, Tudino V, De Leo A, De Vita D, Bortolami M, Scipione L, Pindinello I, Costi R, Di Santo R. Tegaserod for the Treatment of Irritable Bowel Syndrome. Antiinflamm Antiallergy Agents Med Chem. 2020;19:342-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Sinagra E, Morreale GC, Mohammadian G, Fusco G, Guarnotta V, Tomasello G, Cappello F, Rossi F, Amvrosiadis G, Raimondo D. New therapeutic perspectives in irritable bowel syndrome: Targeting low-grade inflammation, immuno-neuroendocrine axis, motility, secretion and beyond. World J Gastroenterol. 2017;23:6593-6627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 53. | Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SS, Sperber A, Spiegel B, Tack J, Vanner S, Walker LS, Whorwell P, Yang Y. Rome IV Diagnostic Questionnaires and Tables for Investigators and Clinicians. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 54. | Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 476] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 55. | Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018;154:1140-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 56. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1226] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 57. | Farrukh A. Measurement of Pain and Related Symptoms in Irritable Bowel Syndrome: The Use of Validated Pain Measurement Tools. Gastrointest Disord. 2022;4:22-29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Raine T, Danese S. Breaking Through the Therapeutic Ceiling: What Will It Take? Gastroenterology. 2022;162:1507-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 81] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 59. | Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2022;71:1117-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 195] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 60. | Zhang T, Zhang C, Zhang J, Sun F, Duan L. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front Cell Infect Microbiol. 2022;12:859967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 61. | Black CJ, Thakur ER, Houghton LA, Quigley EMM, Moayyedi P, Ford AC. Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2020;69:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 62. | Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (1)] |
| 63. | Li X, Li B, Zhang J, Chen T, Wu H, Shi X, Ma J, Qin J, Tang X, Wang F. Efficacy of opioid receptor modulators in patients with irritable bowel syndrome: A systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e24361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Li J, Zhu W, Liu W, Wu Y, Wu B. Rifaximin for Irritable Bowel Syndrome: A Meta-Analysis of Randomized Placebo-Controlled Trials. Medicine (Baltimore). 2016;95:e2534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Black CJ, Burr NE, Camilleri M, Earnest DL, Quigley EM, Moayyedi P, Houghton LA, Ford AC. Efficacy of pharmacological therapies in patients with IBS with diarrhoea or mixed stool pattern: systematic review and network meta-analysis. Gut. 2020;69:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |