Published online Feb 28, 2025. doi: 10.3748/wjg.v31.i8.100146
Revised: December 3, 2024
Accepted: January 13, 2025
Published online: February 28, 2025
Processing time: 167 Days and 14 Hours
The incidence of malignant gastrointestinal (GI) tumors is increasing, and advancements in medical care have significantly improved patient survival rates. As a result, the number of cases involving multiple primary cancers (MPC) has also increased. The rarity of MPC and the absence of sensitive and specific dia
In this article, we report three cases of MPC, each involving malignant tumors of the GI tract as the initial primary carcinoma, offering insights that may aid in effectively managing similar cases.
Patients with GI malignancies face a higher MPC risk. Developing screening and follow-up protocols may enhance detection and treatment outcomes.
Core Tip: Multiple primary cancer (MPC) refers to the presence of two or more distinct primary malignant tumors that arise in either the same organ or multiple separate organs and tissues within a single patient. This article offers a detailing three cases of gastrointestinal malignancies with MPCs. It features a thorough presentation of endoscopic, tomographic, and biopsy images, including both macroscopic and microscopic views. Besides, the article highlights the importance of comprehensive management for patients with MPCs, aiming to raise clinicians' awareness and improve patient outcomes.
- Citation: Bi XR, Zhao SY, Ma YQ, Duan XY, Hu TT, Bi LZ, Cai HY. Multiple primary cancers with gastrointestinal malignant tumors as the first manifestation: Three case reports and review of literature. World J Gastroenterol 2025; 31(8): 100146
- URL: https://www.wjgnet.com/1007-9327/full/v31/i8/100146.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i8.100146
Multiple primary cancer (MPC), also known as duplicated cancer, is characterized by the occurrence of two or more primary malignant tumors, in either a single or multiple noncontiguous organs and tissues within the same patient. While less common than tumor metastasis or recurrence, the incidence of MPC, particularly in gastrointestinal (GI) malignancies, is increasing globally[1,2]. Some of the most recent studies on malignant tumors of the GI tract combined with MPCs are listed in Table 1[3-8]. This trend correlates with the global increase in GI malignancies, as well as more widespread cancer screening and advances in anti-tumor therapies[9]. MPC can easily be mistaken for metastases or tumor recurrence. This confusion often results in underdiagnosis and misdiagnosis, potentially causing patients to miss optimal treatment opportunities. Thus, it is crucial for clinicians to heighten their awareness and vigilance concerning MPC.
| Ref. | Age/sex | Tumors reported |
| Shibata et al[3] | 60/M | Carcinoma of the esophagus/myocardial metastasis |
| Jena and Nundy[4] | 77/M | Esophagus/rectosigmoid/central nervous system |
| Chen et al[5] | 51/M | Pelvis/bladder/colon |
| Zhang et al[6] | 49/W | Colorectal carcinoma/endometrial carcinoma |
| Liu et al[7] | 65/M | Rectal cancer/pelvic classical hodgkin lymphoma |
| Kang et al[8] | 65/M | Esophagus/rectal metastasis |
Internationally, standardized diagnostic criteria for MPC are yet to be fully established, leading to reporting variations between 2.4% and 17% (Table 2). Ragusa et al[10] noted that 75% of patients with MPC are over the age of 50, with a significantly higher incidence in men than in women, largely attributed to smoking or alcohol consumption. Common sites for MPC include the digestive, respiratory, and urinary systems. Previous studies[11-13] have shown that the most frequent first primary cancers in men are gastric, head and neck, esophageal, and colon cancers; whereas in women, they are breast, cervical, and thyroid cancers. Secondary cancers typically involve the lungs and esophagus.
| Time | People/organisations | Events |
| 1889 | Austrian doctor Christian Billroth | Reported the world's first case of MPC |
| 1932 | Warren[14] | First definition of diagnostic criteria for MPC |
| 2004 | International Agency for Research in Oncology/International Association of Cancer Registries | Publication of IARC/IACR standards |
| 2007 | United States National Research Institute | Presentation of SEER criteria |
The world’s first case of MPC was reported by Billroth in 1889. Currently, most countries, including China, utilize the diagnostic criteria for MPC set by Warren[14] in 1932, which require: (1) A malignant pathological diagnosis for each tumor; (2) That each tumor originates from different tissues or organs, exists independently, and is separated by normal tissue; and (3) Confirmation of the absence of metastasis between the tumors. The International Agency for Research on Cancer/International Association of Cancer Registries introduced criteria in 2004[15] that classify MPC based on a 6-month interval, with cases less than 6 months termed synchronous carcinoma (comprising approximately 10% of cases), and those greater than 6 months termed metachronous carcinoma[16]. In 2007, the United States National Cancer Institute introduced the SEER criteria for classifying MPC based on surveillance, epidemiology, and end results[17]. According to these criteria, multiple tumors in the same organ are classified as heterochronous MPC if they occur more than 2 months apart. Only studies conducted in the United States have adopted the SEER standard.
The clinical information and immunohistochemistry results of three patients are summarized in Table 3 and 4.
| Case | Age | Sex | PH | TFH | Multiple primary cancer | SC or MC | Treatment | Outcome | |||
| Symptoms | Location | Pathology | Staging | ||||||||
| 1 | 72 | Man | Smoking > 40 years; No alcohol | + | Abdominal pain; Bloating; Nausea | Colon; Hypopharynx | As-ca; Sc-ca | ⅡB; 0 | MC | Ope | Died |
| 2 | 69 | Man | Smoking > 20 years; Alcohol > 20years; Long-term oral aspirin | - | Abdominal pain; Weight loss; Back pain; Dizziness; Anaemia; Fatigue | Gastric body; Esophagus; Colon | As-ca; Sc-ca; As-ca | ⅠA; ⅠB; ⅡB | SC | Ope (da Vinci); Chemo (XELOX) | Alive |
| 3 | 52 | Woman | No smoking; No alcohol; diabetes; BMI 32.2 | - | Abdominal pain; Bloating; Nausea; Weight loss; Hoarseness; Dysphagia; Anorexia | Gastric body; Esophagus | As-ca; Sc-ca | IIIB; ⅠB | SC | Ope (da Vinci); Chemo (DOX) | Alive |
| Case No. | |||
| 1 | 2 | 3 | |
| CD31 | - | - | + |
| D2-40 | + | - | - |
| S-100 | + | + | + |
| cgA | - | - | - |
| P53 | + | - | + |
| Syn | - | - | - |
| CD56 | + | - | - |
| CDX-2 | + | + | - |
| MLH1 | + | - | + |
| MSH2 | + | + | + |
| MSH6 | + | + | + |
| PMS2 | +/-- | - | + |
| Ki-67 | 75% | 70% | 80% |
| Her-2 | 1+ | - | - |
| Her-2 FISH | - | - | - |
Case 1: A 72-year-old male presented to our hospital in May 2023 with swallowing difficulties.
Case 2: A 69-year-old man sought medical attention in July 2024 due to intermittent abdominal pain.
Case 3: A 52-year-old woman presented to our hospital in February 2023, reporting intermittent epigastric pain for the past year.
Case 1: The patient had a 3-month history a foreign body sensation in his pharynx, swallowing difficulties, poor water intake, worsening hoarseness, and even respiratory effort.
Case 2: The patient had a 1-month history of intermittent abdominal pain, which he described as dull and localized to the right mid-abdomen, even gradually accompanied by difficulty with eating, dizziness, and fatigue.
Case 3: The patient had a 1-year history of persistent subxiphoid swelling and pain. The pain, which worsened after meals, was accompanied by a poor appetite. No other specific discomfort was described.
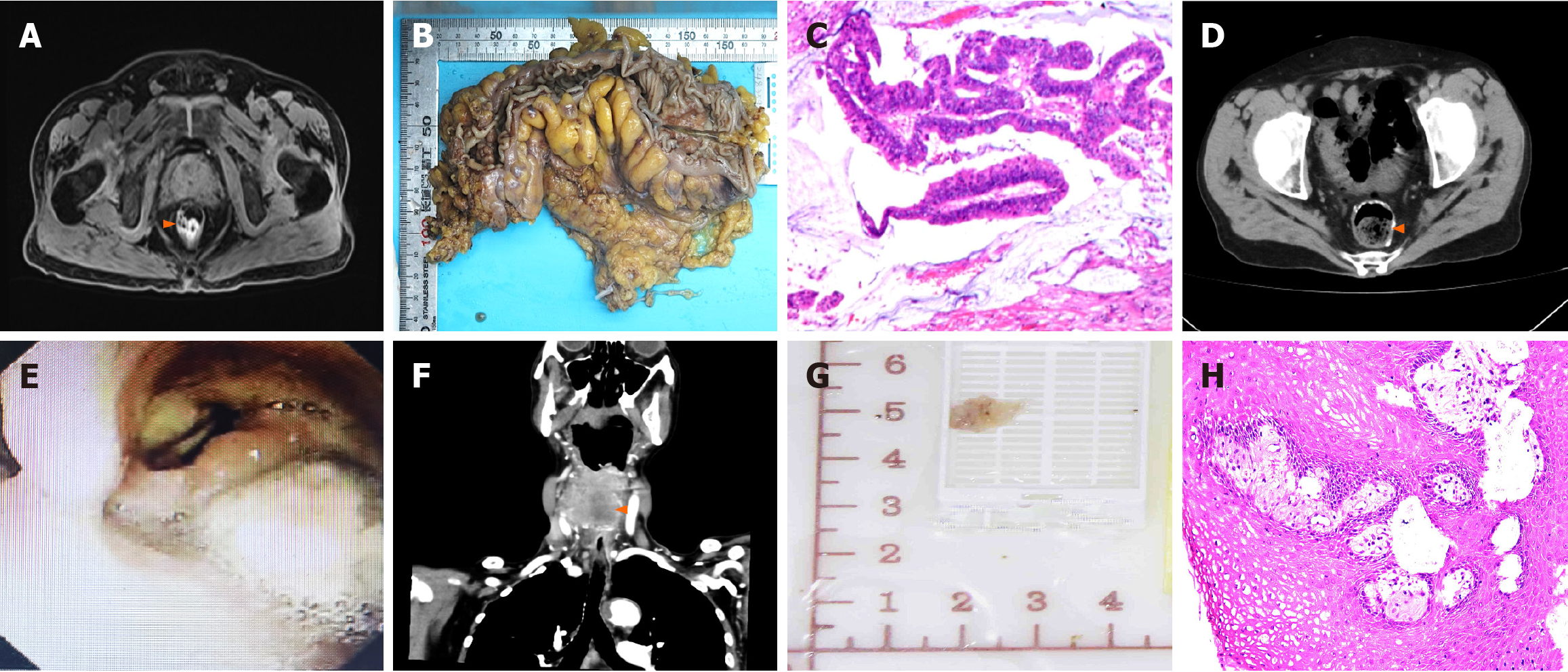
Case 1: The patient was diagnosed with colon cancer in July 2022 and underwent surgical treatment (Figure 1A-D).
Case 2: The patient had coronary stent implantation and was on long-term oral aspirin therapy.
Case 3: The patient had a history of diabetes mellitus.
Case 1: The patient had a 40-year smoking history and no alcohol consumption.
Case 2: The patient had a history of smoking and drinking for more that 20 years.
Case 3: No significant personal or family history was reported.
Case 1: The patient was 172 cm in height and 69.8 kg in weight, with a body mass index (BMI) of 23.6 kg/m2 and recent weight loss of approximately 3 kg. His vital signs were as follows: Consciousness, clear; blood pressure, 119/83 mmHg; heart rate, 77 beats/min; body temperature, 36.2 °C; and respiratory rate, 20 breaths/min. A digital rectal examination was negative.
Case 2: The patient was 168 cm in height and 56.5 kg in weight, with a BMI of 20 kg/m2 and had lost 2 kg in the past 3 months. His vital signs were as follows: Consciousness, clear; blood pressure, 105/72 mmHg; heart rate, 89 beats/min; body temperature, 36.4 °C; and respiratory rate, 18 breaths/min. Notable tenderness were observed in the right mid-abdomen and no masses were touched. There was no palpable spleen or liver, and no nodes were palpable. A digital rectal examination was negative.
Case 3: The patient was 160 cm in height and 82.5 kg in weight, with a BMI of 32.2 kg/m2 and recent weight loss of approximately 1 kg. Her vital signs were as follows: Consciousness, clear; blood pressure, 129/97 mmHg; heart rate, 94 beats/min; body temperature, 36.5 °C; and respiratory rate, 19 breaths/min. All other physical examinations were unremarkable.
Case 1: Laboratory examinations revealed that the patient's carcinoembryonic antigen level was elevated to 6.20 ng/mL, and C-reactive protein level was elevated to 12.59 mg/L. The patient's pro-gastrin-releasing peptide and Squamous epithelial cell carcinoma antigen levels were elevated to 79.90 pg/mL and 4.00 ng/mL, respectively.
Case 2: Laboratory examinations showed a hemoglobin concentration of 92 g/L, a pepsinogen I concentration of 21.6 ng/mL, and positive occult blood in the stool.
Case 3: Laboratory examinations revealed no abnormalities.
Case 1: Laryngoscopy (Figure 1E) revealed a neoplasm with a rough surface was noted on the right lateral pharyngeal wall. This mass extended into the posterior pharyngeal wall and the right pyriform fossa, displacing the laryngopharynx to the left. Neck computed tomography (CT) (Figure 1F) revealed a hypopharyngeal mass with invasion of the right thyroid lobe, cricoid cartilage, and thyroid cartilage, leading to a diagnosis of hypopharyngeal carcinoma.
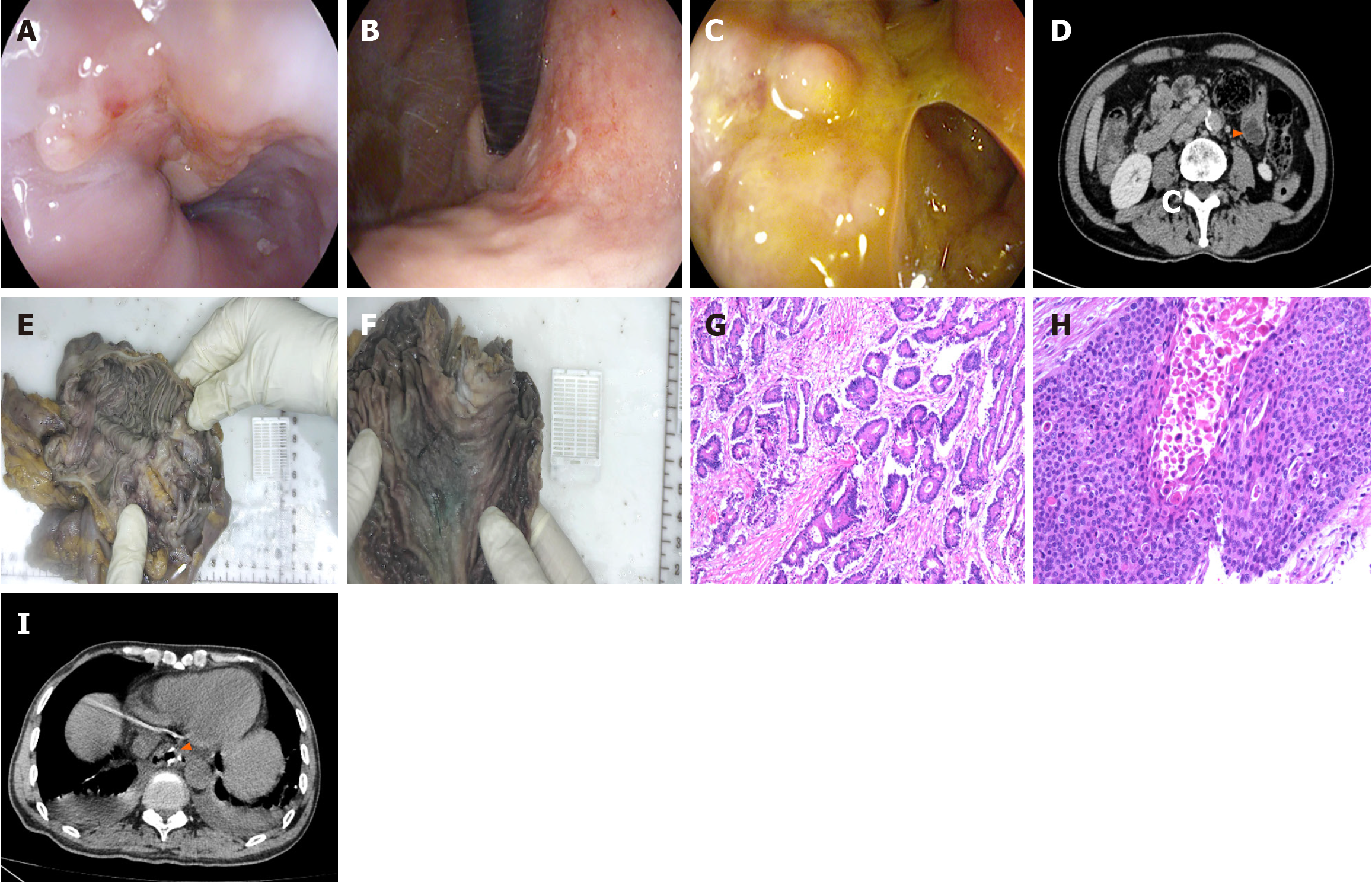
Case 2: Imaging included gastroendoscopy (Figure 2A-C) and abdominal CT (Figure 2D). Abdominal CT revealed a mass in the ascending colon and colohepatic flexure, with multiple regional lymph node metastases in the surrounding mesentery.
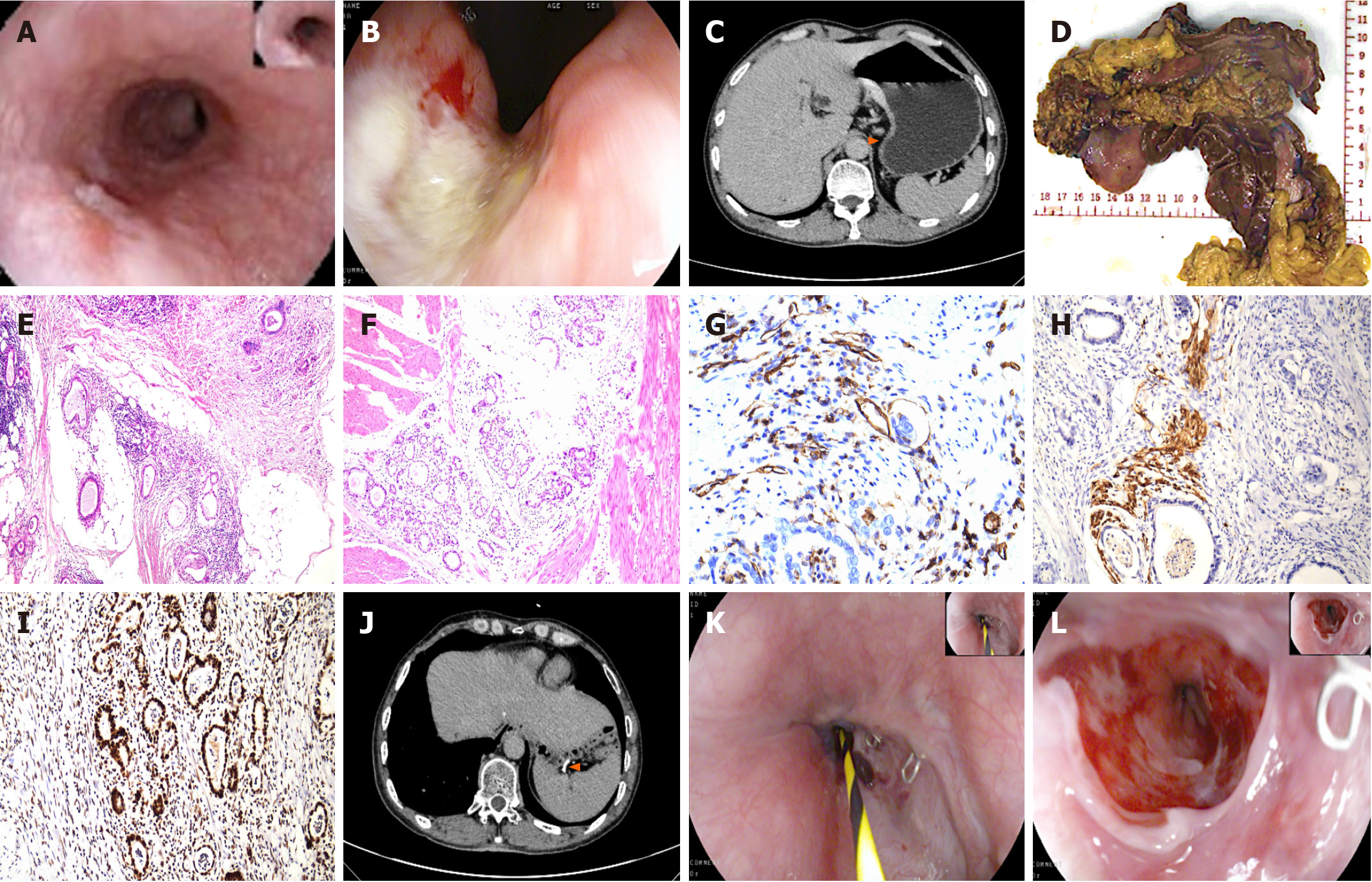
Case 3: Upon admission, gastroscopy was performed (Figure 3A and B). Abdominal CT (Figure 3C) revealed an eccentric wall thickening of the upper esophageal segment and focal wall thickening of the lesser curvature of the stomach, strongly suggestive of a neoplastic lesion.
The intraoperative gross specimen (Figure 1G) and postoperative pathology results (Figure 1H) showed squamous epithelial foci in the right pyriform fossa with high-grade intraepithelial neoplasia (carcinoma in situ). The immunohistochemistry results are detailed in Table 4.
The intraoperative gross specimen (Figure 2E and F) and postoperative pathology results (Figure 2G and H) identified MPCs, namely: (1) Esophageal cancer (moderately differentiated squamous cell carcinoma); (2) Early-stage gastric carcinoma (flat type [type IIb], highly differentiated adenocarcinoma), and (3) Right hemicolon carcinoma (ulcerative type, moderately differentiated adenocarcinoma). No lymph node involvement was noted. The immunohistochemistry results are detailed in Table 4.
The intraoperative gross specimen (Figure 3D) showed a normal mucosal tissue interval between two cancer foci. The postoperative pathology results (Figure 3E and F) revealed gastric cancer of the ulcerative type, with moderately differentiated adenocarcinoma, which had invaded the entire gastric wall. Additionally, a moderately differentiated squamous carcinoma was identified in the esophagus. No lymph node involvement was noted. The immunohistochemistry image results and detailed data are presented in Figure 3G-I and Table 4, respectively.
Despite recommendations for radiation therapy, the patient’s family declined further treatment, leading to his discharge.
To address the anemia and ensure readiness for surgery, a preoperative blood transfusion was administered. The patient then underwent a successful da Vinci robotic-assisted laparoscopic total gastrectomy with esophageal-jejunal anastomosis and right hemicolectomy under general anesthesia. And the current treatment plan includes postoperative chemotherapy with oxaliplatin and capecitabine.
Following the exclusion of surgical contraindications, the patient underwent a da Vinci robotic-assisted laparoscopic total gastrectomy and radical esophagectomy for esophageal cancer. Subsequently, the patient has completed six cycles of chemotherapy using an oxaliplatin, docetaxel, and tiglio regimen.
The patient's repeat CT (Figure 1H) at six months after surgery for hypopharyngeal carcinoma indicated irregular soft tissue masses with unclear boundaries. Although we recommended further treatment, but the patient 's family refused. Finally, the patient passed away 13 months after the first surgery for hypopharyngeal.
The patient's postoperative review of total abdominal CT (Figure 2I) suggested total gastrectomy and postoperative changes in the colon. The patient’s condition continues to be closely monitored with ongoing follow-up.
The patient made a good recovery and was discharged on the 7th postoperative day. His postoperative review of total abdominal CT (Figure 3J) and endoscopy (Figure 3K and L) revealed changes consistent with those expected following gastrectomy, with no significant abnormalities noted. During follow-up, the patient continues to show good overall survival and has no evidence of recurrence.
MPC is a rare clinical entity. Recent studies have identified several factors contributing to the etiology of MPC, including: (1) Genetic factors; (2) Medical-origin factors; and (3) Lifestyle and environmental factors. The development of MPC is strongly associated with genetic mutations. Patients with a family history of cancer have a significantly higher risk of developing additional cancers compared to the general population. Previous studies[18] have highlighted the roles of germline mutations, DNA methylation, microsatellite instability, and single nucleotide polymorphisms in the progression of MPC. These genetic markers may offer pathways for early detection, prevention, and effective treatment[19]. Medical-origin factors include cellular damage and reduced immunity from the use of non-steroidal anti-inflammatory drugs, chemotherapeutic agents, and radiation therapy. While some studies[20-24] have suggested minimal impact of radiation therapy on the incidence of secondary cancers, asserting a potentially lower risk compared to those who have not undergone such treatment, others[25-29] have indicated a potential for increased recurrence. Poor lifestyle choices, such as smoking, excessive alcohol consumption, and obesity, also significantly contribute to the development of MPC[30]. Alcohol use notably heightens the risk of MPC in the upper respiratory and GI tracts, and these effects are magnified when combined with smoking, increasing the risk of gastric cancer patients by 78%[31]. Additionally, an unhealthy diet and obesity are linked to a 3.39-fold increase in the risk of secondary cancers in patients with gastric cancer, with a high BMI correlating with a 2.3-fold increased risk compared to a normal BMI[32,33]. Here, we report three cases of MPC, each involving malignant tumors of the GI tract as the initial primary carcinoma. Case 1 involved colon adenocarcinoma and squamous carcinoma of the hypopharynx. The patient had a significant family history of cancer and a long history of smoking, which likely contributed to the development of MPC. Case 2 presented complex risk factors, including smoking, alcohol consumption, a history of cardiac stent implantation, and chronic use of oral aspirin, all of which may have played a role in the development of MPC. Case 3 involved a woman with a BMI of 32.2, which heightened her risk of gastric cancer associated with MPC.
Given the low incidence of MPC and the absence of specific diagnostic markers, several clinical practices are essential for effective management. First, diagnosis and differential diagnosis are important. An accurate diagnosis is crucial to prevent missed treatment opportunities. Unlike metastatic cancers, which are typically treated palliatively with systemic chemotherapy due to poor prognoses, MPC should be managed according to the specific treatment principles for each primary tumor. Evidence[34,35] indicates that the prognosis for patients with MPC depends more on the pathological type and tumor stage than the number of tumor sites. Surgical resection is often feasible and preferable when the patient’s health and tumor characteristics permit, potentially offering outcomes comparable to or better than those observed in patients with single primary cancers. Secondly, comprehensive assessment and monitoring should be performed. At both the initial diagnosis and during follow-up, it is vital for clinicians to utilize a combination of physical examinations, imaging, and pathology to fully assess the patient’s condition. This approach should include vigilance for tumor recurrence and the possibility of synchronous primary tumors within the GI tract. Multidisciplinary consultations may be necessary to ensure precise diagnosis and treatment. Early detection, diagnosis, and intervention are crucial for improving long-term survival and prognosis in patients with MPCs. Third, the patients should undergo standardized screening and follow-up. Establishing a standardized screening and monitoring system for high-risk groups is crucial. The field of MPC requires more extensive clinical studies and detailed follow-up investigations to gather additional epidemiological data and biological samples. This research is necessary to further explore potential susceptibility genes and epidemiological risk factors, ultimately aiding in the development of improved screening and treatment strategies for MPC. We believe that, with the development of a new generation of molecular sequencing methods, the characteristics of MPCs can be clearly defined and sex gene expression can be more specifically targeted.
The management of patients with MPCs is a concern. Physicians are often faced with the challenging task of selecting appropriate drugs and determining the timing of chemotherapy when different tumors require different treatment regimens. Therefore, we advocate for a comprehensive approach, including conducting numerous relevant clinical trials and promptly establishing clinical guidelines for the diagnosis and treatment of MPC within the international medical community. Moreover, based on the current average economic status of patients, we found that few of them actively request genetic testing, even though genetic testing can be of great utility before the disease occurs, especially for patients with a family history of cancer. Therefore, we call on policymakers to introduce corresponding policies to include genetic testing and genetic analysis into the scope of proportional reimbursement, which will greatly reduce the economic burden of patients.
| 1. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9951] [Article Influence: 4975.5] [Reference Citation Analysis (2)] |
| 2. | Aydiner A, Karadeniz A, Uygun K, Tas S, Tas F, Disci R, Topuz E. Multiple primary neoplasms at a single institution: differences between synchronous and metachronous neoplasms. Am J Clin Oncol. 2000;23:364-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Shibata Y, Ohmura H, Komatsu K, Sagara K, Matsuyama A, Nakano R, Baba E. Myocardial metastasis from ZEB1- and TWIST-positive spindle cell carcinoma of the esophagus: A case report. World J Gastroenterol. 2024;30:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (7)] |
| 4. | Jena SS, Nundy S. Synchronous multiple primary neoplasms of the esophagus, rectosigmoid and central nervous system. Int J Surg Case Rep. 2024;117:109566. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 5. | Chen J, Huang HY, Zhou HC, Liu LX, Kong CF, Zhou Q, Fei JM, Zhu YM, Liu H, Tang YC, Zhou CZ. Three cancers in the renal pelvis, bladder, and colon: A case report. World J Clin Cases. 2024;12:392-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (3)] |
| 6. | Zhang T, Huang X, Liu W, Ling X, Su Z, Huang M, Che S. Rare germline mutation and MSH2-&MSH6 + expression in a double primary carcinoma of colorectal carcinoma and endometrial carcinoma: a case report. Diagn Pathol. 2024;19:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (1)] |
| 7. | Liu S, Li H, Dong Y, Zhang D. Synchronous multiple primary cancers involving rectal cancer and pelvic classical hodgkin lymphoma: the first case report. Front Oncol. 2023;13:1295533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 8. | Kang M, Zhu L, Yang M, Zhang Y, Wang S, Wang Y. Rare presentation of esophageal squamous cell carcinoma with rectal metastasis: A case report. Oncol Lett. 2023;26:510. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 9. | Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10:e0125754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Ragusa R, Torrisi A, Di Prima AA, Torrisi AA, Ippolito A, Ferrante M, Madeddu A, Guardabasso V. Cancer Prevention for Survivors: Incidence of Second Primary Cancers and Sex Differences-A Population-Based Study from an Italian Cancer Registry. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 11. | Si L, Feng Y, Wang Y, Zhong J, Sun Z, Li X, Sun Y. Clinical and pathological characteristics of multiple primary malignant neoplasms cases. Int J Clin Pract. 2021;75:e14663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 12. | Wang H, Hou J, Zhang G, Zhang M, Li P, Yan X, Ma Z. Clinical characteristics and prognostic analysis of multiple primary malignant neoplasms in patients with lung cancer. Cancer Gene Ther. 2019;26:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, Chen Z, Li P, Zhang X, Li S, Yang J. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96:e6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 14. | Warren S. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J cancer. 1932;16:1358-1414. |
| 15. | Working Group Report. International rules for multiple primary cancers (ICD-0 third edition). Eur J Cancer Prev. 2005;14:307-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Skelton WP 4th, Ali A, Skelton MN, Federico R, Bosse R, Nguyen TC, Dang LH, Bishnoi R. Analysis of Overall Survival in Patients With Multiple Primary Malignancies: A Single-center Experience. Cureus. 2019;11:e4552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2018. National Cancer Institute, Bethesda, MD 20892. US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. 2018. Available from: https://seer.cancer.gov/. |
| 18. | Zhang WL, Zhu ZL, Huang MC, Tang YJ, Tang YL, Liang XH. Susceptibility of Multiple Primary Cancers in Patients With Head and Neck Cancer: Nature or Nurture? Front Oncol. 2019;9:1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Cui Y, Ren W, Du X, Yang L, Tan B. Research Progress of Multiple Primary Malignancies Associated With Esophageal Cancer. Cancer Control. 2023;30:10732748231176641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (1)] |
| 20. | Wong LY, Kapula N, He H, Guenthart BA, Vitzthum LK, Horst K, Liou DZ, Backhus LM, Lui NS, Berry MF, Shrager JB, Elliott IA. Risk of developing subsequent primary lung cancer after receiving radiation for breast cancer. JTCVS Open. 2023;16:919-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 21. | Tiruye T, David R, O'Callaghan M, FitzGerald LM, Higgs B, Kahokehr AA, Roder D, Beckmann K. Risk of secondary malignancy following radiation therapy for prostate cancer. Sci Rep. 2023;13:20083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 22. | Chargari C, Goodman KA, Diallo I, Guy JB, Rancoule C, Cosset JM, Deutsch E, Magne N. Risk of second cancers in the era of modern radiation therapy: does the risk/benefit analysis overcome theoretical models? Cancer Metastasis Rev. 2016;35:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Rombouts AJM, Hugen N, Elferink MAG, Feuth T, Poortmans PMP, Nagtegaal ID, de Wilt JHW. Incidence of second tumors after treatment with or without radiation for rectal cancer. Ann Oncol. 2017;28:535-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Pithadia KJ, Advani PG, Citrin DE, Bekelman JE, Withrow DR, Berrington de Gonzalez A, Morton LM, Schonfeld SJ. Comparing Risk for Second Primary Cancers After Intensity-Modulated vs 3-Dimensional Conformal Radiation Therapy for Prostate Cancer, 2002-2015. JAMA Oncol. 2023;9:1119-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 25. | van Leeuwen FE, Ng AK. Long-term risk of second malignancy and cardiovascular disease after Hodgkin lymphoma treatment. Hematology Am Soc Hematol Educ Program. 2016;2016:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Turcotte LM, Liu Q, Yasui Y, Henderson TO, Gibson TM, Leisenring W, Arnold MA, Howell RM, Green DM, Armstrong GT, Robison LL, Neglia JP. Chemotherapy and Risk of Subsequent Malignant Neoplasms in the Childhood Cancer Survivor Study Cohort. J Clin Oncol. 2019;37:3310-3319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 27. | Jin MC, Qian ZJ, Megwalu UC. Risk of Second Primary Malignancies After External Beam Radiotherapy for Thyroid Cancer. Anticancer Res. 2022;42:1359-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 28. | Wen L, Zhong G, Ren M. Increased risk of secondary bladder cancer after radiation therapy for endometrial cancer. Sci Rep. 2022;12:1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 29. | Wakeford R, Hauptmann M. The risk of cancer following high, and very high, doses of ionising radiation. J Radiol Prot. 2022;42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 30. | Li Y, Ye LS, Hu B. Synchronous multiple primary malignancies of the esophagus, stomach, and jejunum: A case report. World J Clin Cases. 2021;9:9889-9895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Reference Citation Analysis (1)] |
| 31. | Jassem J. Tobacco smoking after diagnosis of cancer: clinical aspects. Transl Lung Cancer Res. 2019;8:S50-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 32. | Moke DJ, Hamilton AS, Chehab L, Deapen D, Freyer DR. Obesity and Risk for Second Malignant Neoplasms in Childhood Cancer Survivors: A Case-Control Study Utilizing the California Cancer Registry. Cancer Epidemiol Biomarkers Prev. 2019;28:1612-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Jung SY, Kim YA, Jo M, Park SM, Won YJ, Ghang H, Kong SY, Jung KW, Lee ES. Prediagnosis obesity and secondary primary cancer risk in female cancer survivors: A national cohort study. Cancer Med. 2019;8:824-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Guo Z, Hu L, Chen Q, Hu J, Liu J, Hu W. Synchronous pulmonary MALT lymphoma and squamous cell lung cancer: a case report. World J Surg Oncol. 2023;21:182. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 35. | Dai L, Yang HL, Yan WP, Liang Z, Xiong HC, Kang XZ, Yang YB, Fu H, Fan MY, Chen KN. The equivalent efficacy of multiple operations for multiple primary lung cancer and a single operation for single primary lung cancer. J Thorac Dis. 2016;8:855-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |