Published online Feb 21, 2025. doi: 10.3748/wjg.v31.i7.98448
Revised: November 30, 2024
Accepted: January 2, 2025
Published online: February 21, 2025
Processing time: 204 Days and 20.1 Hours
Endoscopic healing (EH) is a key therapeutic target in Crohn’s disease (CD). Proximal small bowel (SB) lesions in patients with CD are associated with a significant risk of strictures and bowel resection. Assessing SB in patients with CD is necessary because of its significant therapeutic implications. The advent of biologic therapies, including infliximab, ustekinumab, and vedolizumab, has significantly altered CD treatment. However, data on the efficacy of biologics in achieving EH, specifically in the proximal SB of patients with CD, remain limited.
To assess the effectiveness of biologics for EH in patients with jejunal and/or proximal ileal CD.
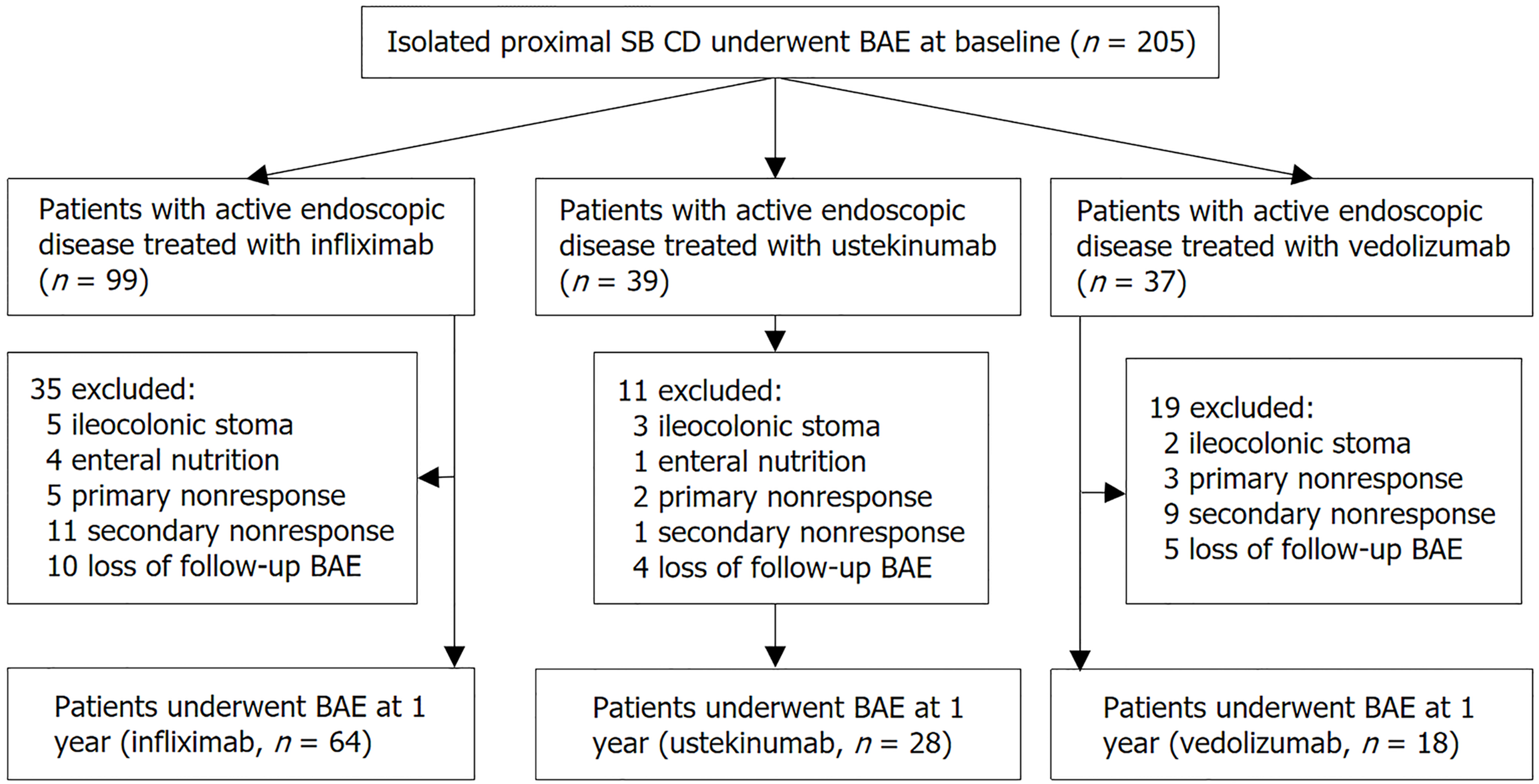
Between 2017 and 2023, we retrospectively included 110 consecutive patients with isolated proximal SB CD, identified through baseline balloon-assisted enteroscopy. These patients completed 1-year of treatment with infliximab, ustekinumab, or vedolizumab, and underwent a second balloon-assisted enteroscopy at 1 year. Com
In total, 64 patients were treated with infliximab, 28 with ustekinumab, and 18 with vedolizumab. The complete EH rate at 1 year was 20.9% (23/110), with 29.6% (19/64) for infliximab, 10.7% (3/28) for ustekinumab, and 5.5% (1/18) for vedolizumab. The median modified SES-CD significantly decreased compared to baseline [5 (2-8) vs 8 (6-9), P < 0.001]. The jejunal and proximal ileal EH rates at 1 year were 30.8% (12/39) and 15.5% (16/103), respectively. Multiple logistic regression analysis showed that stricturing or penetrating disease [odds ratio (OR) = 0.261, 95%CI: 0.087-0.778, P = 0.016], prior exposure to biologics (OR = 0.080, 95%CI: 0.010-0.674, P = 0.020), and moderate-to-severe endoscopic disease (OR = 0.277, 95%CI: 0.093-0.829, P = 0.022) were associated with a lower likelihood of achieving EH at 1 year.
Only 20.9% of patients with isolated proximal SB CD achieved complete EH after 1 year of biologic therapy.
Core Tip: Endoscopic healing (EH) is a key treatment target for Crohn’s disease (CD), but limited data are available on isolated proximal small bowel (SB) disease. This retrospective study analyzed the data of patients with isolated proximal SB CD identified through balloon-assisted enteroscopy. We aimed to evaluate the effectiveness of biologics for EH in patients with jejunal and proximal ileal CD at 1 year. The results showed that 20.9% of patients achieved complete EH after 1-year biologic therapy. Failure to achieve complete EH was significantly associated with stricturing or penetrating disease, prior exposure to biologics, and moderate-to-severe endoscopic disease.
- Citation: Huang ZC, Wang BY, Peng B, Liu ZC, Lin HX, Yang QF, Tang J, Chao K, Li M, Gao X, Guo Q. Effectiveness of biologics for endoscopic healing in patients with isolated proximal small bowel Crohn’s disease. World J Gastroenterol 2025; 31(7): 98448
- URL: https://www.wjgnet.com/1007-9327/full/v31/i7/98448.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i7.98448
Crohn’s disease (CD) is a chronic, progressive, and destructive bowel disease that impacts the entire gastrointestinal system, primarily affecting the terminal ileum and colon[1,2]. The advent of biological therapies, including infliximab, ustekinumab, and vedolizumab, has significantly altered CD treatment. Endoscopic healing (EH) is a key goal in CD management, with numerous studies demonstrating its association with favorable outcomes[3,4]. However, most majority of the available evidence on EH relies on ileocolonoscopic data, which can overlook small bowel (SB) lesions because the disease can skip the distal ileum[5]. Proximal SB involvement occurs in approximately 15% of patients with CD[6,7]. While these proximal SB lesions are less common, they are associated with a higher risk of stricturing or penetrating complications in the course of the disease than colonic or distal ileal CD[8-10]. Therefore, evaluating the proximal SB is essential for CD management due to its significant therapeutic implications. However, data on the efficacy of biologics in achieving EH specifically in the proximal SB of patients with CD, remain limited.
Balloon-assisted enteroscopy (BAE) enables the evaluation of areas of the proximal SB that are not accessible through conventional colonoscopy[11]. Its introduction has significantly enhanced the detection of SB mucosal lesions[12]. Therefore, this study retrospectively analyzed data from patients with isolated proximal SB CD, who underwent BAE after receiving 1 year of treatment with infliximab, ustekinumab, or vedolizumab.
We aimed to evaluate the effectiveness of these biologics for EH in patients with jejunal and proximal ileal CD at 1 year.
This retrospective, longitudinal cohort study was conducted at a single tertiary referral hospital in China. Consecutive adult patients with isolated proximal SB CD, identified through baseline BAE assessments, were included between 2017 and 2023. The inclusion criteria were as follows: (1) Patients only had isolated proximal SB disease without terminal ileal and/or colonic involvement; (2) Presence of at least one segment with a modified Simple Endoscopic Score for CD (SES-CD) of ≥ 3 and any ulcer size upon baseline BAE evaluation; (3) Initiation of therapy with infliximab, ustekinumab, or vedolizumab, and (4) Undergoing a follow-up BAE evaluation 1 year after starting treatment. We excluded patients who had an ileocolonic stoma, were concurrently using exclusive enteral nutrition, or had switched to an alternative treatment before undergoing the second BAE.
The selection of biologics for patients was guided by guidelines and also took into account individual patient preferences. Each biologic was administered following guidelines[13-15]. For infliximab induction, patients followed standard induction (standard doses at weeks 0, 2, and 6) and maintenance (5 mg/kg intravenously every 8 weeks) protocols. For ustekinumab, a single intravenous dose (approximately 6 mg/kg) was administered for induction, fo
This study was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (ID: 2023ZSLYEC-609). Informed consent from patients was waived because the study was retrospective, and all patient data were fully de-identified.
All patients underwent a BAE (EN-450P5 or EN-580T, Fujifilm, Tokyo, Japan). The BAE procedures were performed and evaluated by two experienced endoscopists. We calculated the length of the SB examined by counting the number of over-tube strokes[16]. The SB was divided into 3 segments: The terminal ileum (≤ 30 cm from the ileocecal valve), the proximal ileum (30-300 cm from the ileocecal valve), and the jejunum (the proximal part of SB, excluding the section defined as the proximal ileum and duodenum) (Supplementary Table 1)[15,17]. Isolated proximal SB was defined as an SB > 30 cm away from the ileocecal valve.
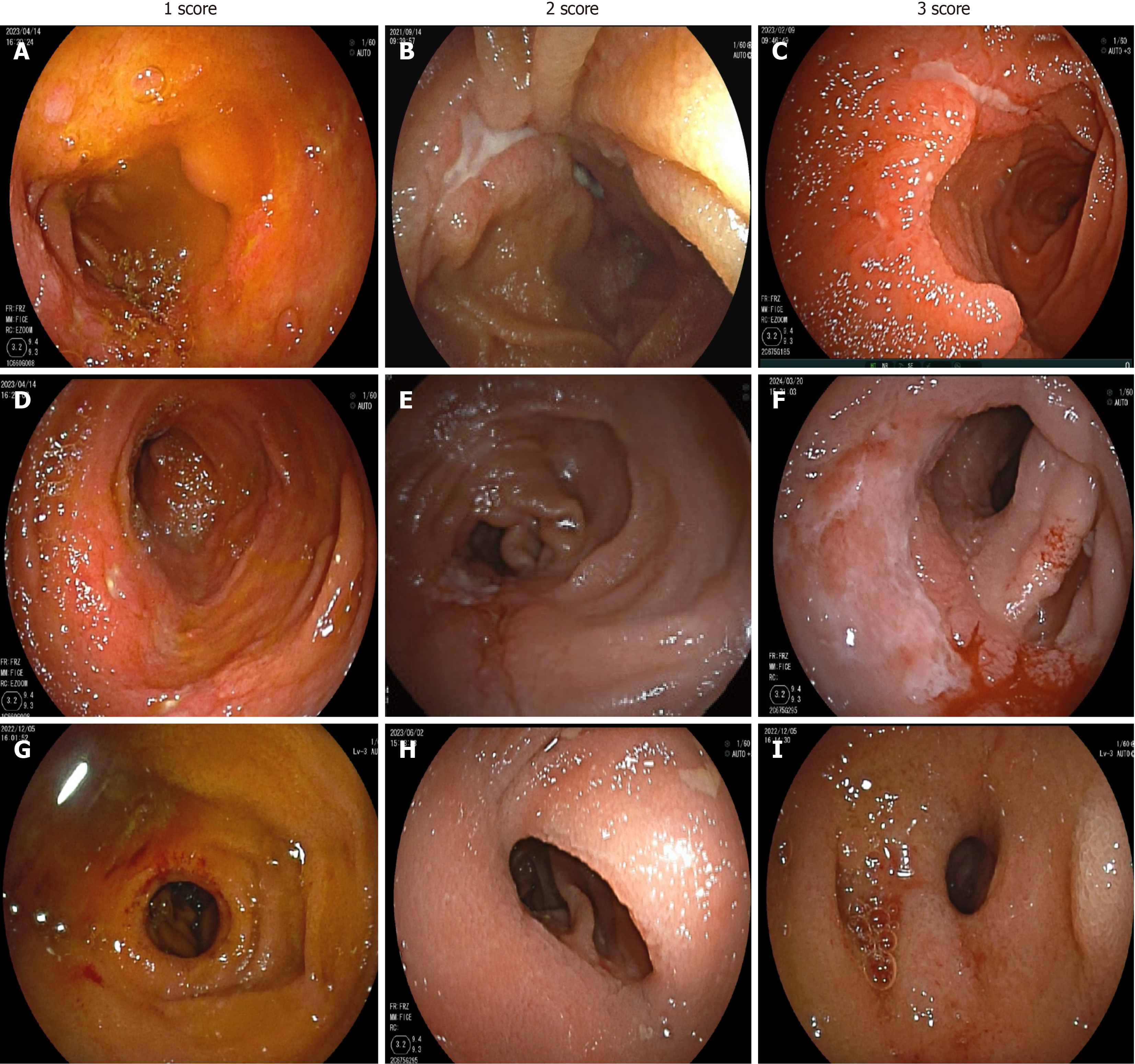
BAE evaluations of the SB were performed using the modified SES-CD. This scoring system assesses SB lesion activity based on four endoscopic variables: The size of ulcers, the proportion of the ulcerated surface, the proportion of the affected surface, and the presence of stenosis. Each variable was scored on a scale of 0-3 for each of the three segments, and the total score for each segment ranged from 0 to 12 (Supplementary Table 1)[17,18]. The overall modified SES-CD score was then calculated as the sum of the scores for each SB segment. The size of ulcers was classified as 0 (none), 1 (small, from 0.1 to < 0.5 cm), 2 (large, from 0.5 to 2 cm), and 3 (very large, > 2 cm). Figure 1 shows the representative BAE images of patients with different modified SES-CD scores.
The primary outcome of this study was to analyze the efficacy of three approved biologics for CD in achieving 1 year of complete EH, defined as a completely modified SES-CD < 3. One-year EH of the jejunum and proximal ileum was separately defined as a modified SES-CD of 0 in the segment assessed[19]. The secondary outcomes were the rate of endoscopic response (ER) rates and complete ulcer healing at 1 year. ER was defined as a reduction in the modified SES-CD score by more than 50% at both the complete and segmental levels[20]. Complete ulcer healing was defined as no inflammatory changes present, including erosions and ulcerations[18].
Descriptive data were presented as mean ± SD or median with interquartile range (IQR). Categorical data were re
Of the 205 patients with active isolated proximal SB CD, 95 patients were excluded for various reasons. Ultimately, we assessed 110 patients who finished 1 year of biologics treatment and underwent a follow-up BAE evaluation after 1 year. The process of patient selection is illustrated in Figure 2. Baseline characteristics are summarized in Table 1. The median age was 33 years (IQR: 25-39), and 88 (80.0%) patients were male. The median disease duration was 1.1 years (IQR: 0.4-2.5), and 33 (30.0%) patients had prior exposure to biologics. The proximal ileum was the most frequently affected bowel segment in proximal SB CD, observed in 103 (93.6%) patients, while jejunal involvement was observed in 39 (35.5%). Stricturing and penetrating diseases were present in 62 (56.4%) and 18 (16.4%) patients, respectively. Regarding biologic treatments, 64 (58.2%), 28 (25.5%), and 18 (16.4%) patients were treated with infliximab, ustekinumab, and vedolizumab, respectively. The baseline characteristics of patients stratified by biologics are summarized in Supplementary Table 2.
| Variables | All patients (n = 110) |
| Male | 88 (80.0) |
| Age, years, median (IQR) | 33 (25-39) |
| Disease duration, years, median (IQR) | 1.1 (0.4-2.5) |
| Smoking history | 18 (16.4) |
| Disease behavior | |
| Bl: Inflammatory | 30 (27.3) |
| B2: Stricturing | 62 (56.4) |
| B3: Penetrating | 18 (16.4) |
| Perianal disease | 65 (59.1) |
| HBI, median (IQR) | 7 (5-7) |
| HBI > 4 | 94 (85.5) |
| CRP, mg/L, median (IQR) | 2.1 (0.6-8.9) |
| Albumin, g/L, median (IQR) | 39 (36-42) |
| Hemoglobin, g/L, median (IQR) | 129 (112-139) |
| Prior biologic exposure | 33 (30.0) |
| Current biologic therapy | |
| Infliximab | 64 (58.2) |
| Ustekinumab | 28 (25.5) |
| Vedolizumab | 18 (16.4) |
| Concomitant treatment | |
| Steroids | 3 (2.7) |
| Immunomodulator | 34 (30.9) |
| Baseline BAE evaluation | |
| Modified SES-CD, median (IQR) | 8 (6-9) |
| Bowel segment with active disease1 | |
| Jejunum | 39 (35.5) |
| Proximal ileum | 103 (93.6) |
| Very large ulcers (> 2.0 cm) | |
| Jejunum | 11/39 (28.2) |
| Proximal ileum | 35/103 (34.0) |
| Dose optimization | 7 (6.4) |
At baseline assessment, all patients underwent transanal and transoral BAE, and the scope reached the entire intestine in 48 (43.6%) patients. Overall, all patients had ulcer lesions at proximal SB, 73 (66.4%) had stricturing disease and 74 (67.3%) had moderate-to-severe endoscopic activity (modified SES-CD ≥ 7). The median total modified SES-CD was 8 (IQR: 6-9). The median segmental modified SES-CD for the jejunum was 4 (IQR: 3-5), and that for the proximal ileum was 6 (IQR: 5-7).
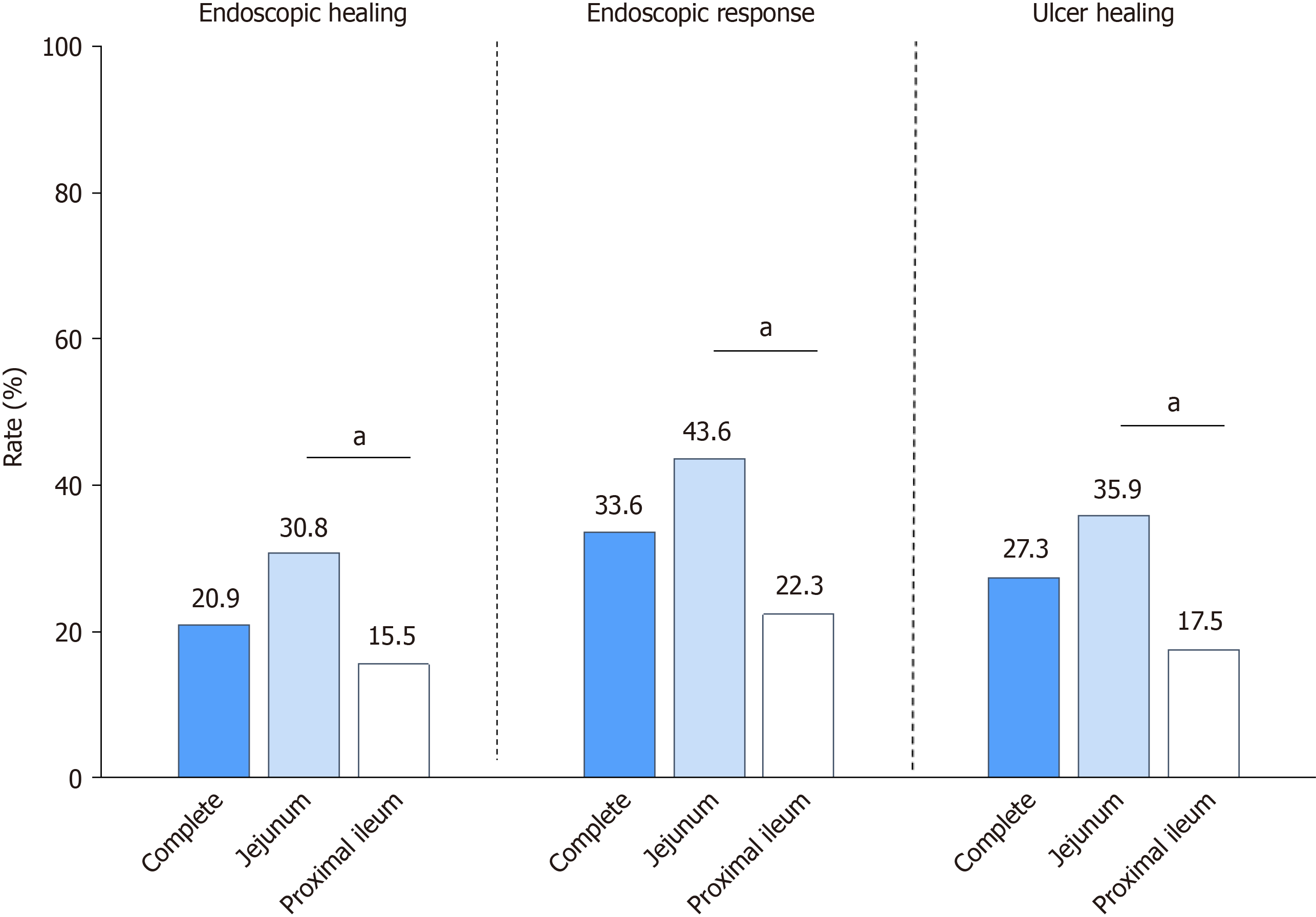
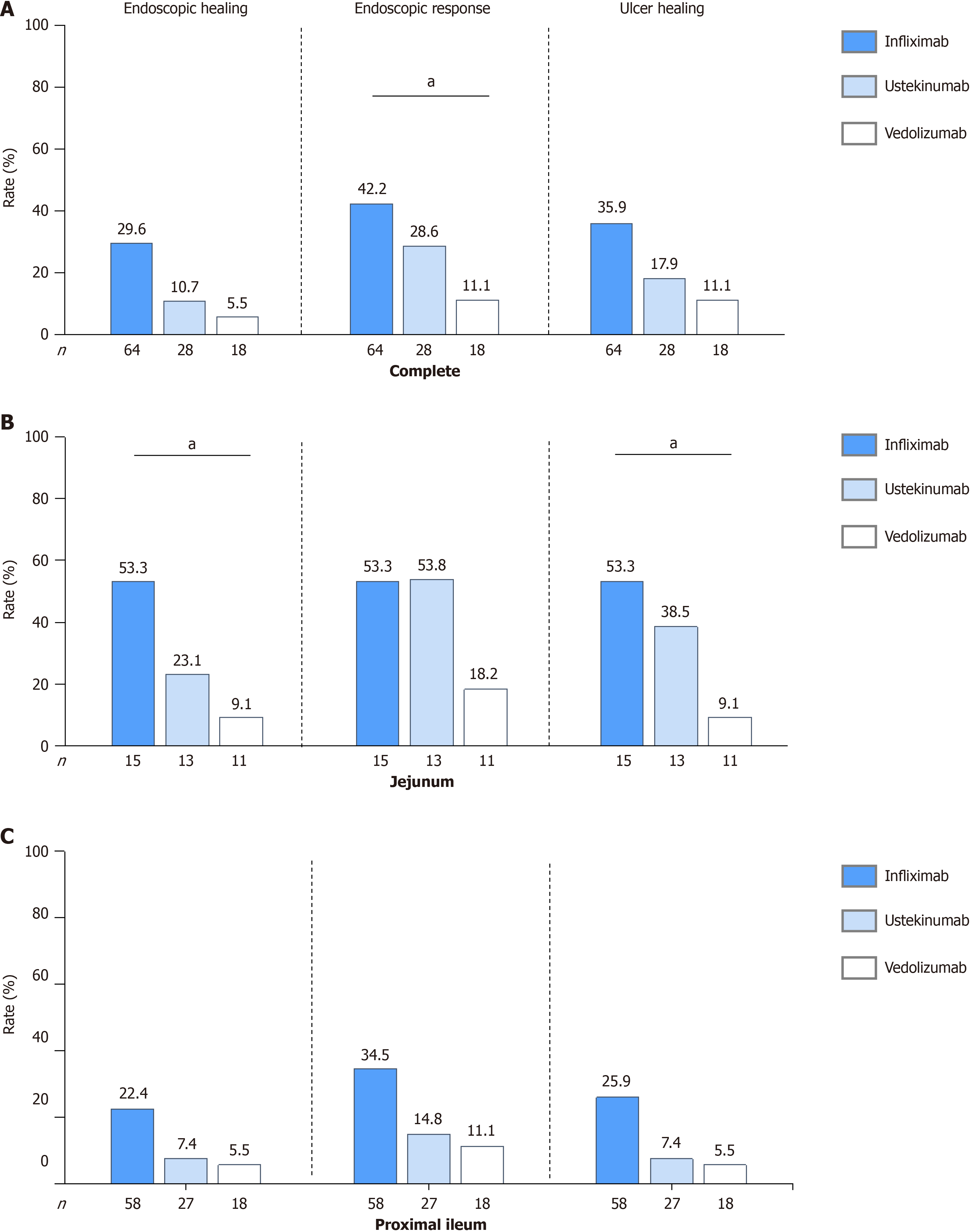
During the second examination, all patients underwent transanal and transoral BAE, and the scope reached the entire intestine in 57 (51.8%) patients. Figure 3 shows that 20.9% (23/110) of the patients achieved complete EH at 1 year. Patients treated with infliximab demonstrated the numerically highest EH rate at 1 year [29.6% (19/64)], followed by those treated with ustekinumab [10.7% (3/28)] and vedolizumab [5.5% (1/18)]. Similar trends were observed for achieving ER. In total, 33.6% (37/110) of patients achieved ER: 42.2% (27/64) in the infliximab-, 28.6% (8/28) in the ustekinumab-, and 11.1% (2/18) in the vedolizumab-treated patients (Figure 4A, Supplementary Table 3). Patients treated with infliximab had a significantly higher ER rate than patients treated with vedolizumab (P = 0.015). The median modified SES-CD significantly decreased at 1 year compared to baseline [5 (IQR: 2-8) vs 8 (IQR: 6-9), P < 0.001].
Figures 3 and 4A summarizes the rates of complete ulcer healing among all patients. At 1 year, the complete ulcer healing rates for patients was 27.3% (30/110). Patients treated with infliximab demonstrated the numerically highest ulcer healing rate at 1 year [35.9% (23/64)], followed by those treated with ustekinumab [17.9% (5/28)] and vedolizumab [11.1% (2/18)]. At baseline, 35 patients had very large ulcers and 49 had large ulcers. The complete ulcer healing rates for patients with very large or larger ulcers were 17.9% (15/84): Very large 22.9% (8/35) and large 14.3% (7/49). Notably, among patients with large or very large ulcers, those treated with infliximab showed a significantly higher complete ulcer healing rate [31.8% (14/44)] than those treated with ustekinumab [4.0% (0/25), P = 0.017] and vedolizumab [0.0% (0/15), P = 0.013]. For detailed complete ulcer healing rates for patients with very large and large ulcers, respectively (Sup
In total, 39 patients had a jejunum with segmental modified SES-CD ≥ 3. Among these, 12 (30.8%) achieved jejunal EH, 17 (43.6%) achieved ER, and 14 (35.9%) achieved ulcer healing at 1 year (Figure 3). Vedolizumab-treated patients demon
Additionally, 103 patients had a proximal ileum with segmental modified SES-CD ≥ 3. Of these, 16 (15.5%) achieved proximal ileal EH, 23 (22.3%) achieved ER, and 18 (17.5%) achieved ulcer healing at 1 year, respectively (Figure 3). Ve
Notably, significant differences were observed in segmental EH rates between the jejunum and proximal ileum [30.8% (12/39) vs 15.5% (16/103), P = 0.042; Figure 3]. Similar trends were observed in segmental ER rates [43.6% (17/39) vs 22.3% (23/103), P = 0.012] and in segmental ulcer healing rate [35.9% (14/39) vs 17.5% (18/103), P = 0.012] between the two segments (Figure 3).
Supplementary Table 4 summarizes the rates of ulcer healing in the jejunum and the proximal ileum after 1 year of biologic therapy. At baseline, 11 patients had very large ulcers in the jejunum and 35 patients in proximal ileum. There was no significant difference in the rate of complete ulcer healing between the jejunum and proximal ileum at 1 year [27.3% (3/11) vs 17.1% (6/35), P = 0.664]. Similar results were observed between the jejunum and proximal ileum which had large ulcers at baseline [27.8% (5/18) vs 15.2% (7/46), P = 0.247].
At 1 year, 67.3% (74/110) of patients were in clinical remission (HBI < 4) and 74.5% (82/110) were in biochemical remission (CRP ≤ 3mg/L). The median HBI significantly decreased at 1 year compared to that at baseline [3 (2-4) vs 7 (5-7), P < 0.001]. The correlation between the total modified SES-CD and HBI scores was weak both at baseline (r = 0.172, P = 0.072) and 1 year (r = 0.214, P < 0.05). Similarly, the median CRP level significantly decreased compared to that at baseline [0.8 (0.5-3.2) vs 2.1 (0.6-8.9) mg/L, P < 0.001]. The correlation between the total modified SES-CD and CRP levels was also weak at both baseline (r = 0.196, P < 0.05) and 1 year (r = 0.160, P = 0.095; Supplementary Figures 2 and 3).
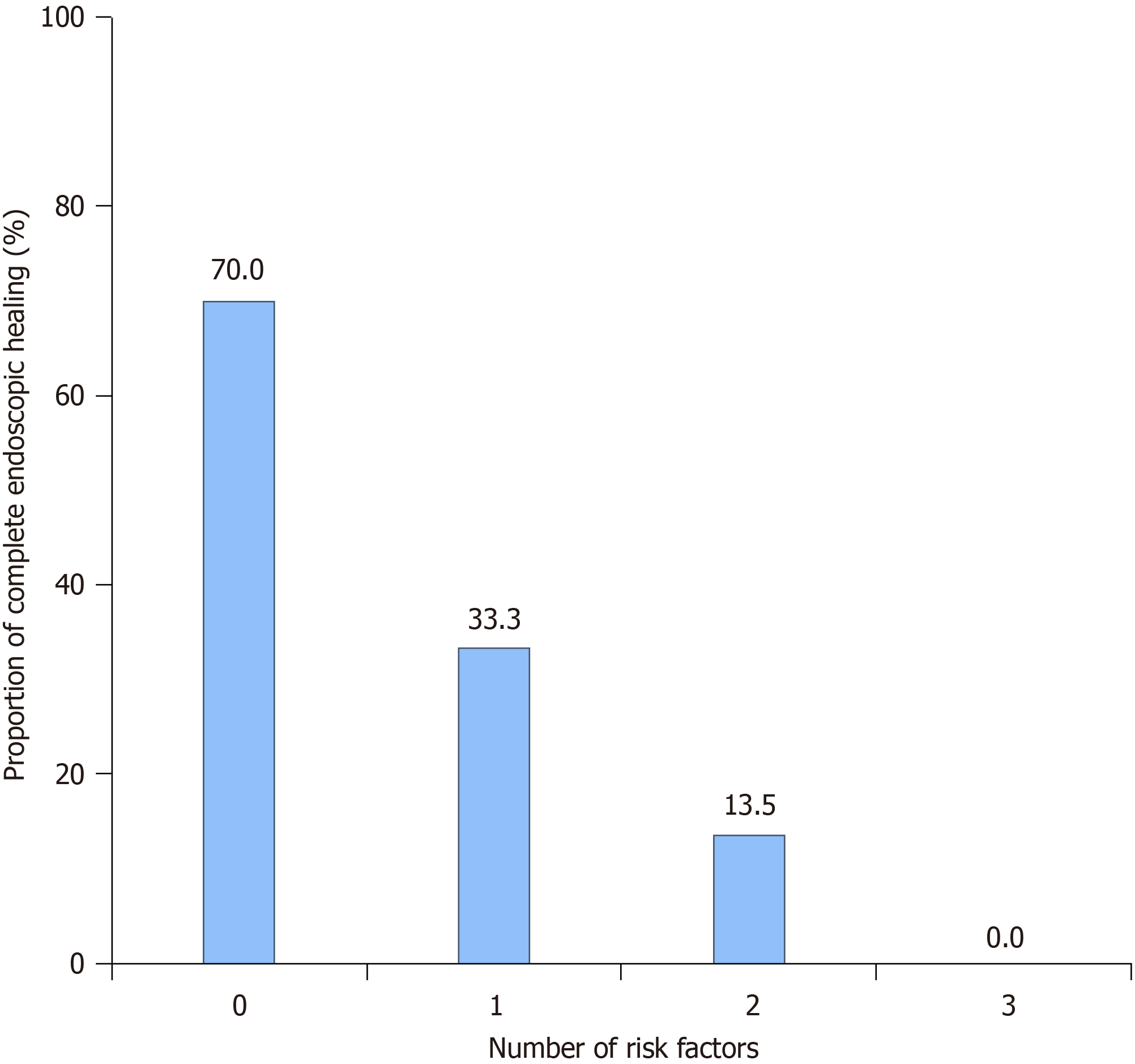
In multivariate logistic regression analysis, the following factors were identified as risk factors for achieving complete EH: Stricturing or penetrating behavior [odds ratio (OR) = 0.261, 95%CI: 0.087-0.778, P = 0.016], prior exposure to biologics (OR = 0.080, 95%CI: 0.010-0.674, P = 0.020), and moderate-to-severe endoscopic disease (OR = 0.277, 95%CI: 0.093-0.829, P = 0.022; Table 2). The rates of complete EH were 70.0% (7/10) for the patients with none of these risk factors, 33.3% (5/15) for 1 factor, 13.5% (5/37) for 2 factors, and 0 for 3 factors (Figure 5). With each added risk factor, the likelihood of achieving complete EH decreases by 76.1%, as indicated by an OR of 0.239 (95%CI: 0.112-0.509, P < 0.001).
| Variables | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | aOR | 95%CI | P value | |
| Male | 1.863 | 0.500-6.944 | 0.354 | |||
| Age > 40 | 0.457 | 0.097-2.161 | 0.323 | |||
| Disease duration > 2 years | 0.578 | 0.207-1.612 | 0.295 | |||
| Active smoker | 0.423 | 0.090-1.988 | 0.276 | |||
| B2/3 behavior | 0.191 | 0.066-0.559 | < 0.001 | 0.261 | 0.087-0.778 | 0.016 |
| Perianal involvement | 0.434 | 0.156-1.207 | 0.434 | |||
| Prior biologic exposure | 0.078 | 0.010-0.607 | 0.015 | 0.080 | 0.010-0.674 | 0.020 |
| Immunomodulator use | 0.271 | 0.075-0.985 | 0.047 | 0.448 | 0.108-1.860 | 0.269 |
| Moderate-to-severe endoscopic disease1 | 3.617 | 1.396-9.374 | 0.008 | 0.277 | 0.093-0.829 | 0.022 |
| Prior intestinal resection | 0.350 | 0.042-2.886 | 0.329 | |||
| Dose optimization | 1.630 | 0.186-14.256 | 0.659 | |||
| Type of biological therapies (IFX reference) | ||||||
| Vedolizumab | 0.139 | 0.017-1.123 | 0.064 | |||
| Ustekinumab | 0.533 | 0.277-1.027 | 0.060 | |||
No serious adverse events were observed in all patients during the use of the 3 different biologics. Only two patients treated with infliximab experienced adverse reactions. One patient had a positive T-SPOT test at the 24 weeks of assessment, but pulmonary computed tomography and other examinations did not show tuberculosis activation, hence isoniazid was administered orally for prophylactic anti-tuberculosis treatment, and continued infliximab treatment. The other case was a definite pulmonary infection. After regular anti-infection treatment, the patient recovered and was allowed to continue infliximab treatment.
To our knowledge, this is the first study to evaluate the effectiveness of biologics in achieving EH in patients with isolated proximal SB CD. Our findings revealed that only 20.9% of patients with isolated proximal SB CD achieved complete EH after 1 year of biological therapy. At segmental level analyses, 30.8% and 15.5% of the jejunum and proximal ileum, respectively, achieved segmental EH at 1 year. Additionally, failure to achieve complete EH was significantly associated with stricturing or penetrating disease, prior exposure to biologics, and moderate-to-severe endoscopic disease. Our study underscores the nuanced challenges in achieving EH in isolated proximal SB CD, emphasizing the need for tailored treatment strategies that consider individual patient risk factors and disease characteristics.
Several cohort studies have suggested that SB lesions do not heal as well as the colon after receiving biological therapy[21,22]. Indeed, many studies have primarily focused on lesions in the terminal ileum and colon, thus potentially over
After 1 year of biological treatment, the complete EH rate in patients with isolated proximal SB CD was 20.9% (23/110) and the ER rate was 33.6% (37/110). Takenaka et al[18] reported that 36% (41/114) of patients had SB EH during infliximab maintenance therapy based on BAE evaluation. Similarly, a subsequent study by Han et al[24] revealed SB endoscopic remission rate of 38% (41/108) and EH rate of 27.8% (30/108) after treatment with infliximab. Our rates are relatively low compared to those reported in several previous studies[25-27]. However, previous studies primarily uti
Predictors of failure to achieve EH have been reported in several studies. De Cruz et al[28] identified factors like young age at diagnosis, the presence of a perianal fistula, delayed treatment, and smoking as predictors of poor outcomes. Freeman[29] further reported that complicated disease behavior and proximal SB involvement are also associated with poor outcomes. Our study found that stricturing or penetrating disease, prior exposure to biologics, and moderate-to-severe endoscopic disease were significantly associated with the failure to achieve EH in patients with proximal SB CD. With each added risk factor, the likelihood of achieving complete EH decreases by 76.1%. Notably, 77.3% (85/110) of patients presented with two or more risk factors, likely contributing to the poor response observed in a large percentage of our study population. Furthermore, previous research has also indicated that proximal SB lesions are typically associated with poorer prognoses[7]. Proximal SB CD, particularly with jejunal lesions, is more likely to develop into stricturing or penetrating disease, typically resulting in a poorer prognosis[8,30]. Thus, early intervention, before the pro
Our results also indicate that neither HBI nor CRP levels adequately reflect the endoscopic disease activity in SB CD, aligning with previous studies[32]. Previous research has suggested a poor consistency between fecal calprotectin levels and SB lesions[33]. Therefore, using BAE to monitor the ER in patients with isolated proximal SB CD is clinically im
This study had a few limitations. Despite reaching statistical significance, the relatively small sample size and uneven baselines of various biologics limited the accuracy of comparisons. Additionally, some patients did not undergo follow-up BAE after 1 year of treatment, and others dropped out early because of treatment ineffectiveness or adverse effects. Finally, we did not compare lesions at other sites of CD, as this was not the focus of the current study.
This study advances our understanding of the effectiveness of biologic therapy for patients with isolated proximal SB CD, highlighting the distinct challenges in achieving EH. Our findings reveal a relatively low EH rate of 20.9% in this cohort, which is significantly associated with stricturing or penetrating disease, prior exposure to biologics, and moderate-to-severe endoscopic disease. Early intervention before the development of complicated stricturing or penetrating disease, along with therapeutic drug monitoring and combination therapies, may improve endoscopic outcomes in SB CD. Additionally, infliximab seemingly displayed superior effectiveness in achieving endoscopic improvement than uste
| 1. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2377] [Article Influence: 158.5] [Reference Citation Analysis (1)] |
| 2. | Cholapranee A, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn's disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn's disease. Aliment Pharmacol Ther. 2016;43:317-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 4. | Dulai PS, Levesque BG, Feagan BG, D'Haens G, Sandborn WJ. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointest Endosc. 2015;82:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Samuel S, Bruining DH, Loftus EV Jr, Becker B, Fletcher JG, Mandrekar JN, Zinsmeister AR, Sandborn WJ. Endoscopic skipping of the distal terminal ileum in Crohn's disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol. 2012;10:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4108] [Article Influence: 513.5] [Reference Citation Analysis (110)] |
| 7. | Kim OZ, Han DS, Park CH, Eun CS, Kim YS, Kim YH, Cheon JH, Ye BD, Kim JS. The Clinical Characteristics and Prognosis of Crohn's Disease in Korean Patients Showing Proximal Small Bowel Involvement: Results from the CONNECT Study. Gut Liver. 2018;12:67-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Lazarev M, Huang C, Bitton A, Cho JH, Duerr RH, McGovern DP, Proctor DD, Regueiro M, Rioux JD, Schumm PP, Taylor KD, Silverberg MS, Steinhart AH, Hutfless S, Brant SR. Relationship between proximal Crohn's disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Du J, Du H, Chen H, Shen L, Zhang B, Xu W, Zhang Z, Chen C. Characteristics and prognosis of isolated small-bowel Crohn's disease. Int J Colorectal Dis. 2020;35:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Guo H, Tang J, Qin X, Lin M, Li M, Yang Q, Huang Z, Gao X, Chao K. A novel location classification system for Crohn's disease based on small bowel involvement: a better predictor of disease progression. Gastroenterol Rep (Oxf). 2024;12:goae003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, Naganuma M, Araki A, Watanabe M. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn's disease. Gastroenterology. 2014;147:334-342.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 861] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 13. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1568] [Article Influence: 130.7] [Reference Citation Analysis (1)] |
| 14. | Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P; UNITI-IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. N Engl J Med. 2016;375:1946-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1345] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 15. | Inflammatory Bowel Disease Group; Chinese Society of Gastroenterology; Chinese Medical Association. Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J Dig Dis. 2021;22:298-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 16. | Ning SB, Yang H, Li B, Zhang Y, Huang S, Peng B, Lin H, Kurban M, Li M, Guo Q. Balloon-assisted enteroscopy-based endoscopic stricturotomy for deep small bowel strictures from Crohn's disease: First cohort study of a novel approach. Dig Liver Dis. 2023;55:1397-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, Fujioka T, Matsuoka K, Naganuma M, Watanabe M. Correlation of the Endoscopic and Magnetic Resonance Scoring Systems in the Deep Small Intestine in Crohn's Disease. Inflamm Bowel Dis. 2015;21:1832-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Takenaka K, Fujii T, Suzuki K, Shimizu H, Motobayashi M, Hibiya S, Saito E, Nagahori M, Watanabe M, Ohtsuka K. Small Bowel Healing Detected by Endoscopy in Patients With Crohn's Disease After Treatment With Antibodies Against Tumor Necrosis Factor. Clin Gastroenterol Hepatol. 2020;18:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Narula N, Wong ECL, Dulai PS, Marshall JK, Jairath V, Reinisch W. Comparative Effectiveness of Biologics for Endoscopic Healing of the Ileum and Colon in Crohn's Disease. Am J Gastroenterol. 2022;117:1106-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Vuitton L, Marteau P, Sandborn WJ, Levesque BG, Feagan B, Vermeire S, Danese S, D'Haens G, Lowenberg M, Khanna R, Fiorino G, Travis S, Mary JY, Peyrin-Biroulet L. IOIBD technical review on endoscopic indices for Crohn's disease clinical trials. Gut. 2016;65:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn's disease. Aliment Pharmacol Ther. 2018;48:394-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 22. | Esaki M, Sakata Y. Clinical Impact of Endoscopic Evaluation of the Small Bowel in Crohn's Disease. Digestion. 2023;104:51-57. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: a systematic review of isolated colonic Crohn's disease: the third IBD? Gut. 2017;66:362-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Han W, Hu J, Wu J, Zhang P, Liu Q, Hu N, Mei Q. Use of double-balloon endoscopy and an endoscopic scoring system to assess endoscopic remission in isolated small bowel Crohn's disease after treatment with infliximab. Therap Adv Gastroenterol. 2024;17:17562848231224842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Rivière P, D'Haens G, Peyrin-Biroulet L, Baert F, Lambrecht G, Pariente B, Bossuyt P, Buisson A, Oldenburg B, Vermeire S, Laharie D. Location but Not Severity of Endoscopic Lesions Influences Endoscopic Remission Rates in Crohn's Disease: A Post Hoc Analysis of TAILORIX. Am J Gastroenterol. 2021;116:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 26. | Narula N, Wong ECL, Aruljothy A, Dulai PS, Colombel JF, Marshall JK, Ferrante M, Reinisch W. Ileal and Rectal Ulcer Size Affects the Ability to Achieve Endoscopic Remission: A Post hoc Analysis of the SONIC Trial. Am J Gastroenterol. 2020;115:1236-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Danese S, Sandborn WJ, Colombel JF, Vermeire S, Glover SC, Rimola J, Siegelman J, Jones S, Bornstein JD, Feagan BG. Endoscopic, Radiologic, and Histologic Healing With Vedolizumab in Patients With Active Crohn's Disease. Gastroenterology. 2019;157:1007-1018.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 28. | De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM, Bampton PA, Gibson PR, Sparrow M, Leong RW, Florin TH, Gearry RB, Radford-Smith G, Macrae FA, Debinski H, Selby W, Kronborg I, Johnston MJ, Woods R, Elliott PR, Bell SJ, Brown SJ, Connell WR, Desmond PV. Crohn's disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 444] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 29. | Freeman HJ. Long-term clinical behavior of jejunoileal involvement in Crohn's disease. Can J Gastroenterol. 2005;19:575-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Sunada K, Shinozaki S, Nagayama M, Yano T, Takezawa T, Ino Y, Sakamoto H, Miura Y, Hayashi Y, Sato H, Lefor AK, Yamamoto H. Long-term Outcomes in Patients with Small Intestinal Strictures Secondary to Crohn's Disease After Double-balloon Endoscopy-assisted Balloon Dilation. Inflamm Bowel Dis. 2016;22:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Takenaka K, Kawamoto A, Hibiya S, Suzuki K, Fujii T, Motobayashi M, Shimizu H, Nagahori M, Saito E, Okamoto R, Watanabe M, Ohtsuka K. Higher concentrations of cytokine blockers are needed to obtain small bowel mucosal healing during maintenance therapy in Crohn's disease. Aliment Pharmacol Ther. 2021;54:1052-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol. 2010;8:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 332] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 33. | Koulaouzidis A, Sipponen T, Nemeth A, Makins R, Kopylov U, Nadler M, Giannakou A, Yung DE, Johansson GW, Bartzis L, Thorlacius H, Seidman EG, Eliakim R, Plevris JN, Toth E. Association Between Fecal Calprotectin Levels and Small-bowel Inflammation Score in Capsule Endoscopy: A Multicenter Retrospective Study. Dig Dis Sci. 2016;61:2033-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Bettenworth D, Bokemeyer A, Kou L, Lopez R, Bena JF, El Ouali S, Mao R, Kurada S, Bhatt A, Beyna T, Halloran B, Reeson M, Hosomi S, Kishi M, Hirai F, Ohmiya N, Rieder F. Systematic review with meta-analysis: efficacy of balloon-assisted enteroscopy for dilation of small bowel Crohn's disease strictures. Aliment Pharmacol Ther. 2020;52:1104-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |