Published online Feb 21, 2025. doi: 10.3748/wjg.v31.i7.97599
Revised: November 25, 2024
Accepted: December 18, 2024
Published online: February 21, 2025
Processing time: 230 Days and 3.9 Hours
Radiation enteritis is a common complication of radiation therapy in which the surrounding normal intestinal tissue is damaged by ionising radiation, and there is no standard pharmacological prophylaxis or treatment regimen available. Mesenchymal stem cell transplantation can be used for radiation protection and the treatment of acute radiation injury, but its therapeutic mechanism of action remains unclear.
To investigate the protective effects of autologous bone marrow-derived me
A model of acute radioactive enteritis was established in dogs by applying abdominal intensity-modulated radiation at a single X-ray dose of 12 Gy. ABMSCs were transplanted into the mesenteric artery with the technology of femoral artery puncture and DSA imaging two days after radiation. Visual and histopathological changes of the experimental dogs were observed. Different kinds of cytokines from intestinal samples were tested using Quantibody Canine Cytokine Array method. Enzyme-linked immunosorbent assay (ELISA) was also used to evaluate the cytokines changes in serum.
The ABMSCs group showed significant improvements in survival status compared with the blank and saline treatment groups. Histological observations revealed that the former had lower histological scores than the later after treatment (P < 0.05). Compared to the control groups, interleukin (IL)-10 and monocyte chemotactic protein (MCP)-1 from intestinal samples showed a remarkable increase and ELISA of serum samples proved higher secretion of the two target cytokines in the ABMSCs group (P < 0.05).
Our data suggest that transplantation of ABMSCs promotes intestinal recovery after acute radioactive injury in Beagle dogs. The cytokines of IL-10 and MCP-1 might play an important role in this process.
Core Tip: Mesenchymal stem cell transplantation has a therapeutic role in intestinal radiation injury, but the exact mechanism is unknown. In the present study, we explored the therapeutic role of autologous bone marrow-derived mesenchymal stem cells (ABMSCs) and their possible mechanisms in a Beagle model of radioactive intestinal injury. Our experimental results suggest that ABMSC transplantation has a protective effect against acute radiation enteritis in Beagle dogs and that interleukin-10 and monocyte chemotactic protein-1 may play an important role in the treatment of ABMSCs.
- Citation: Sun GC, Xu WD, Yao H, Chen J, Chai RN. Protective effects of autologous bone marrow-derived mesenchymal stem cell transplantation on acute radioactive enteritis in Beagle dogs. World J Gastroenterol 2025; 31(7): 97599
- URL: https://www.wjgnet.com/1007-9327/full/v31/i7/97599.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i7.97599
Radiation injury can be induced by nuclear radiation or radiotherapy and acute exposure can lead to a range of tissue damage that can be life-threatening[1]. Radiation therapy for abdominal or pelvic cancers and moderate-dose exposure to radiation may damage intestinal tissue that can affect patient’s quality of life. Acute radioactive enteritis may result in epithelial damage, loss of intestinal barrier function, and even lethal sepsis[2]. However, there are no satisfactory medical treatments for intestinal injury after radiotherapy or radiation, which is just treated based on symptoms[3].
Transplantation of mesenchymal stem cells (MSCs) is used for the radiation protection and therapy of acute radiation in several reports[3,4]. MSCs can produce many kinds of cytokines and regulate the capacity of T cell migration and apoptosis through expressing monocyte chemotactic protein (MCP)-1[5]. Meanwhile, MSCs have the ability of secreting interleukin (IL)-10 and other immunomodulatory and paracrine factors which are involved in tissue repair of different organs[6]. IL-10 and MCP-1 are thought to play an important role in anti-inflammation by mobilizing partner cytokines.
So far, the mechanism of MSC transplantation to induce a therapeutic benefit in intestinal radioactive damage is still unclear. In this study, we investigated the effects of autologous bone marrow-derived MSC (ABMSC) transplantation on radiation-induced intestinal injury in Beagle dogs. We found that survival rate and histological observations improved greatly after ABMSC transplantation, which prompted that ABMSCs could induce the regeneration of the intestinal epithelium and regulate the secretion of cytokines in serum and intestine tissue. These findings could be beneficial in the development of novel and effective mitigators of and protectors against acute radiation enteritis.
From March 2022 to September 2023, 15 Beagle dogs (age: 21-27months; weight: 14.37 ± 0.73 kg; mixed breed) were obtained from Department of Animal Experiments, General Hospital of Northern Theater Command. All dogs were treated in compliance with recommendations of Animal Care Committee of General Hospital of Northern Theater Command (permit number: SYXK[2023-15]). This study was approved by the local institution ethics committee. Anesthesia (propofol; 0.5 mL/kg; Northeast Pharmaceutical, Shenyang, China) was used during bone marrow collection, irradiation, endoscopic performance, and transplantation treatment. After irradiation exposure, experimental dogs were treated with antibiotics and parenteral alimentation. Oral Yunnan Baiyao and Lyophilizing Thrombin Power were provided to animals after radiation if hemorrhage and/or bloody stools occurred.
Fifteen dogs were randomly divided into three groups: Blank control group, saline treatment group, and ABMSCs treatment group. Abdominal irradiation was performed on anesthetized saline treatment group and ABMSCs treatment group dogs using intensity-modulated radiation therapy. In addition, ADAC (Pinnacle 3.0) treatment planning system was used for dose calculation before X-ray irradiation. The acceptance criteria made sure that 95% of the doses covered at least 99% of the planning target volume, so the damage was concentrated on the small intestine and damages to the surrounding organs were avoided. In our study, dogs in the saline treatment group and ABMSCs treatment group received abdominal irradiation on the planning target volume at 12 Gy using a 6 MeV liner accelerator (Siemens Primus, Munich, Germany).
Bone marrow cells were obtained from the tibial plateau of healthy dogs 2-3 weeks before irradiation. As previously described[4], BMSCs were isolated by Percoll density centrifugation (Pharmacia, NJ, United States) and were cultured in MSC basal medium (HyClone, Logan, UT, United States) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, United States), 1% glutamine, and 1% penicillin/streptomycin. The cultures were maintained at 37 °C in an atmosphere containing 5% CO2 and subcultured. The nonadherent cells were removed by a medium change at 48-72 hours and every 3-4 days thereafter. After 8-10 days, cells were lifted by incubation with trypsin/super paramagnetic iron oxide (SPIO) and then were expanded. BMSCs were collected for transplantation at a concentration of 1 × 108 cells/mL.
Forty-eight hours post abdominal radiation, transplantation of ABMSCs was administered. ABMSCs were injected into the mesenteric artery by catheterization via femoral artery puncture using Seldinger technology with a 4.0F Cobra catheter (Terumo, Japan). Animals in the ABMSCs treatment group were treated with ABMSCs at 4 × 107 cells/kg, while animals in the saline treatment group were treated with 0.9% normal saline.
Before and after ABMSC transplantation, Beagle dogs’ weight and rectal temperature were recorded every day. A series of symptoms including nausea, vomiting, diarrhea, and bloody stool were also observed and recorded daily. The survival time was compared between different groups.
Endoscopy (EC-450ZW, EC590-ZW/M, Fujinon, Tokyo, Japan) was performed at the time points as follows: Before irradiation, 1 day post irradiation, and 7 days post treatment. Oral administration of polyethylene glycol solution was used 1 day before endoscopy examination. Equal numbers of small intestine biopsies were obtained from dogs at approximately the same distance from the ileocecus. After irradiation and transplantation, tissues were obtained from disease-affected areas by endoscopy.
Two days after ABMSC transplantation, magnetic resonance imaging (MRI) was performed. SPIO-labeled ABMSCs showed low signal (grey) on T2-weighted images, and could be recognized compared with peripheral intestinal segments. But in T1-weighted images, they could not be recognized. MRI was conducted using a GE 3.0 T magnetic resonance scanner with an 8-channel phased array coil. The scan protocol incuded TSE sequence, gradient field intensity 50 mT/m, and gradient switching rate 150 T/m/second. Location scan, axial scan, coronal scan, and sagittal T1W1/T2W2 scan were performed, respectively. The scanning parameters were: Scanning time, 30 minutes; scanning field (FOV), 380 mm × 380 mm; repetition time, 1400 milliseconds; and echo time, 93 milliseconds.
After washing 5 times with 0.9% saline, intestinal samples taken by endoscopy were cut into suitable sizes for fixing in 4% formalin and 2.5% glutaraldehyde solutions. A paraffin slicing machine (CUT4062, SLEE, Mainz, Germany) was used for preparin paraffin-embedded tissue sections (4 μm). Some tissue samples were quickly put into the liquid nitrogen tank and/or re-embedded in Tissue OCT-Freeze Medium and then stored at -80 °C. After fixation in 4% paraformaldehyde for 30 minutes and then washing with PBS, frozen sample sections were added into 2% potassium ferrocyanide and 6% hydrochloric acid (Gene Era Biotech, Hangzhou, China) mixture in the same proportion. After incubation for 30 minutes at 37 °C, PBS was used for washing 3 times. Nuclear red fast solution was used to restrain sample sections for 1 minute. When sample sections were stained with hematoxylin and eosin, they were deparaffined with xylene and hydrated with graded ethanol. We also conducted Prussian blue staining following the manufacturer’s protocol. The histologic characteristics of hematoxylin and eosin stained intestinal samples were evaluated by histologic scores[7]. Specific scores of mucosa damage, inflammatory infiltration, and vascular congestion were set from 0 (absent) to 4 (maxim of each aspect, ++++), and the total score was calculated from 0 to 12.
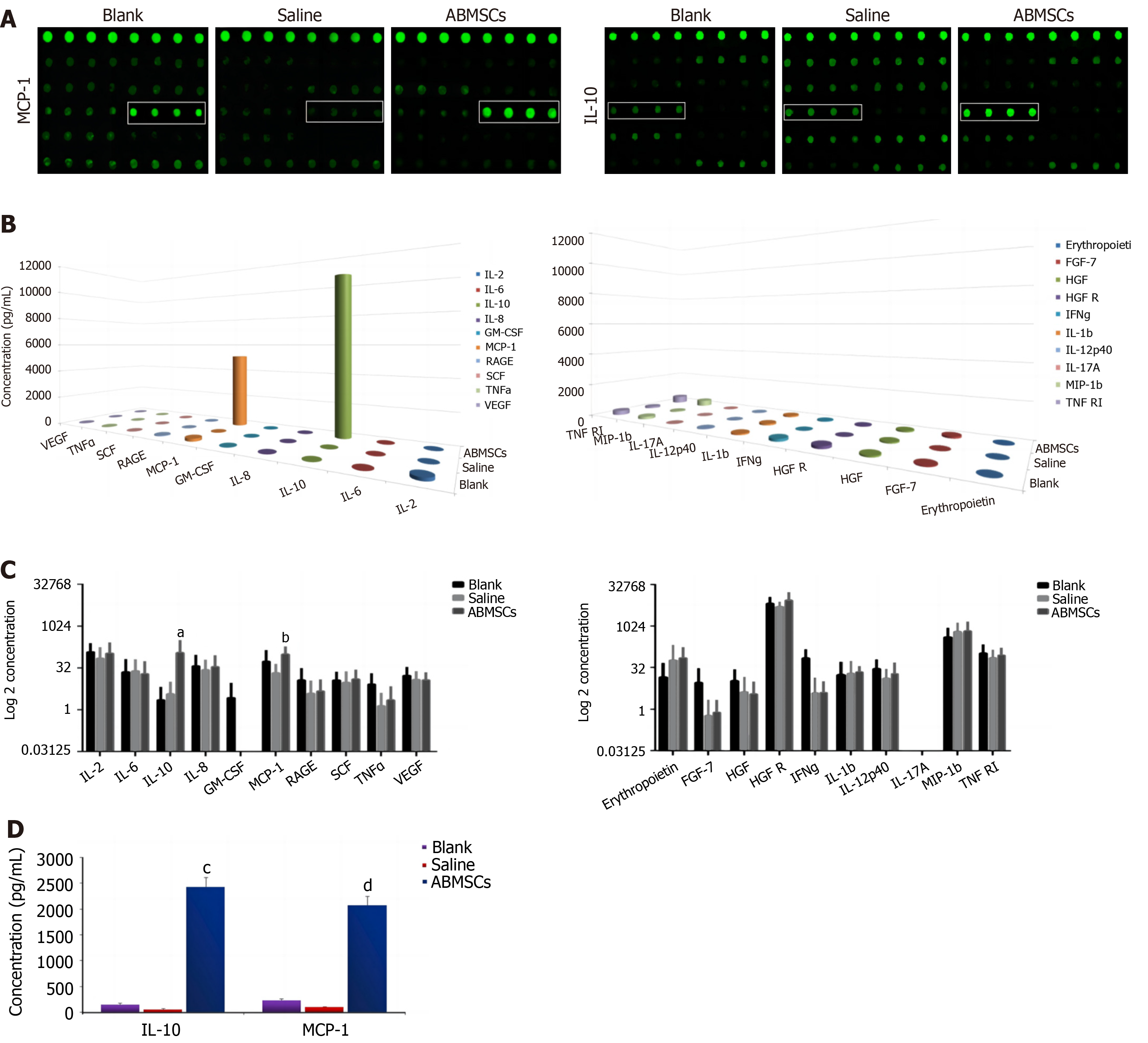
Quantibody® Canine Cytokine Array (RayBiotech, Norcross, GA, United States) analysis was applied to detect the inflammatory cytokines IL-2, IL-6, IL-8, IL-10, granulocyte-macrophage colony stimulating factor (GM-CSF), MCP-1, receptor for advanced glycation end products (RAGE), stem cell factor (SCF), tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF), erythropoietin, fibroblast growth factor (FGF)-7, hepatocyte growth factor (HGF), HGF receptor (HGFR), interferon (IFN)-γ, IL-1β, IL-12p40, IL-17A, MIP-1β, and TNF receptor superfamily member 1A (TNF RI) in intestinal samples according to the manufacturer’s instructions. The intestinal tissue samples of experimental dogs were assayed by enzyme-linked immunosorbent assay (ELISA) to verify the results. Intestinal samples were collected by endoscopy before irradiation, 1 day post irradiation, and 7 days post treatment.
Experimental values are expressed as the mean and standard error of the mean. To compare the results between different groups, a t-test or one-way ANOVA was performed with SPSS17.0 software. Statistical analysis of survival rates in different groups was performed using the Kaplan-Meier Log-rank test. Statistical significance for our analyses was set at P < 0.05.
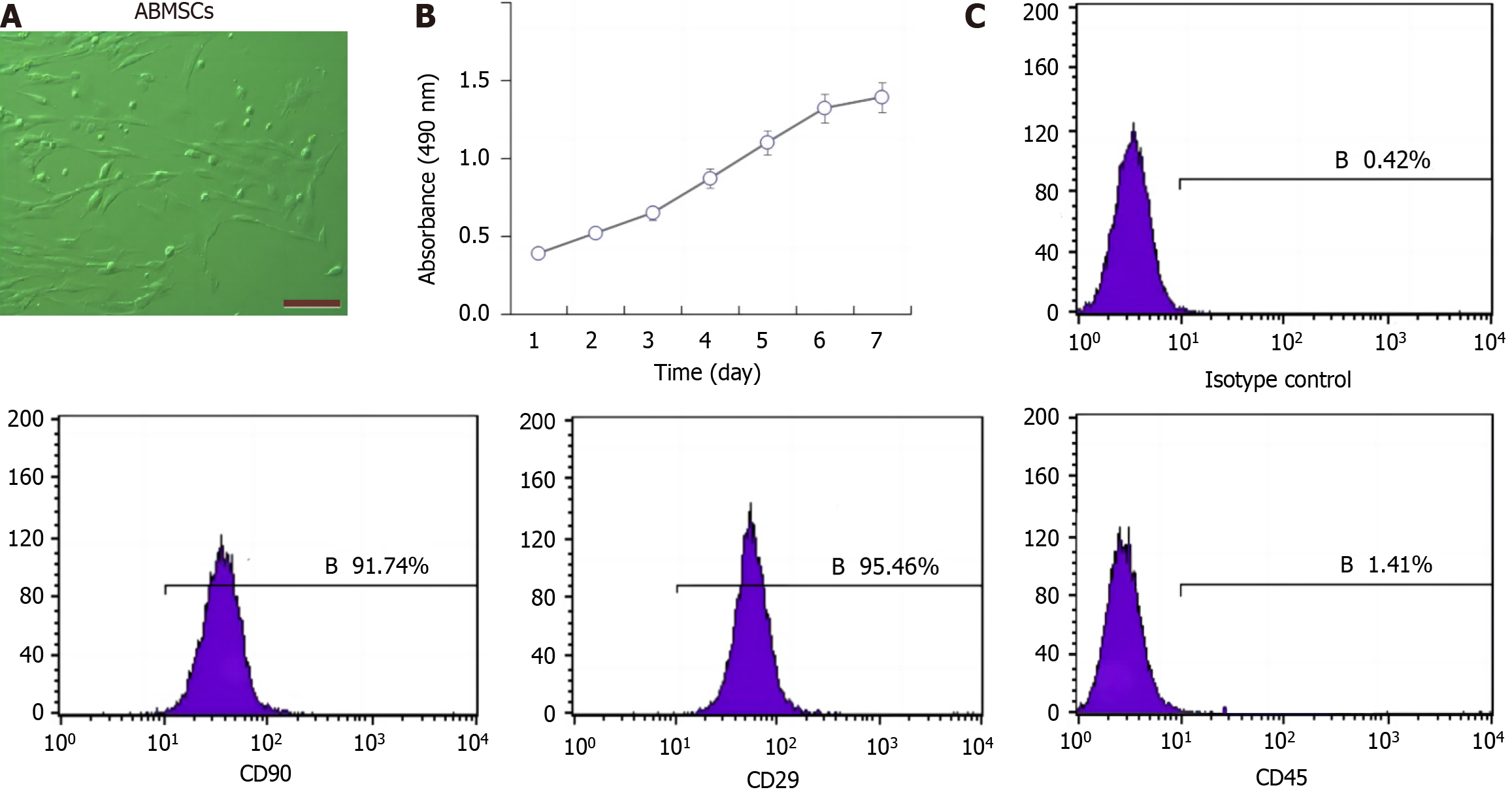
ABMSCs exhibited triangle, polygon, and spindle-shaped appearance which were similar to fibroblasts (Figure 1A). The cell growth curve shows that cell growth was consistent with the results visualized via microscopy (Figure 1B). Flow cytometric data show that the CD90-positive rate was 91.74%, the CD29-positive rate was 95.46%, and the CD45-positive rate was only 1.41% (Figure 1C). Meanwhile, the results of osteogenic differentiation, lipogenic differentiation, and chondrogenic differentiation demonstrated that the extracted cells could be characterized as BMSCs (data not shown).
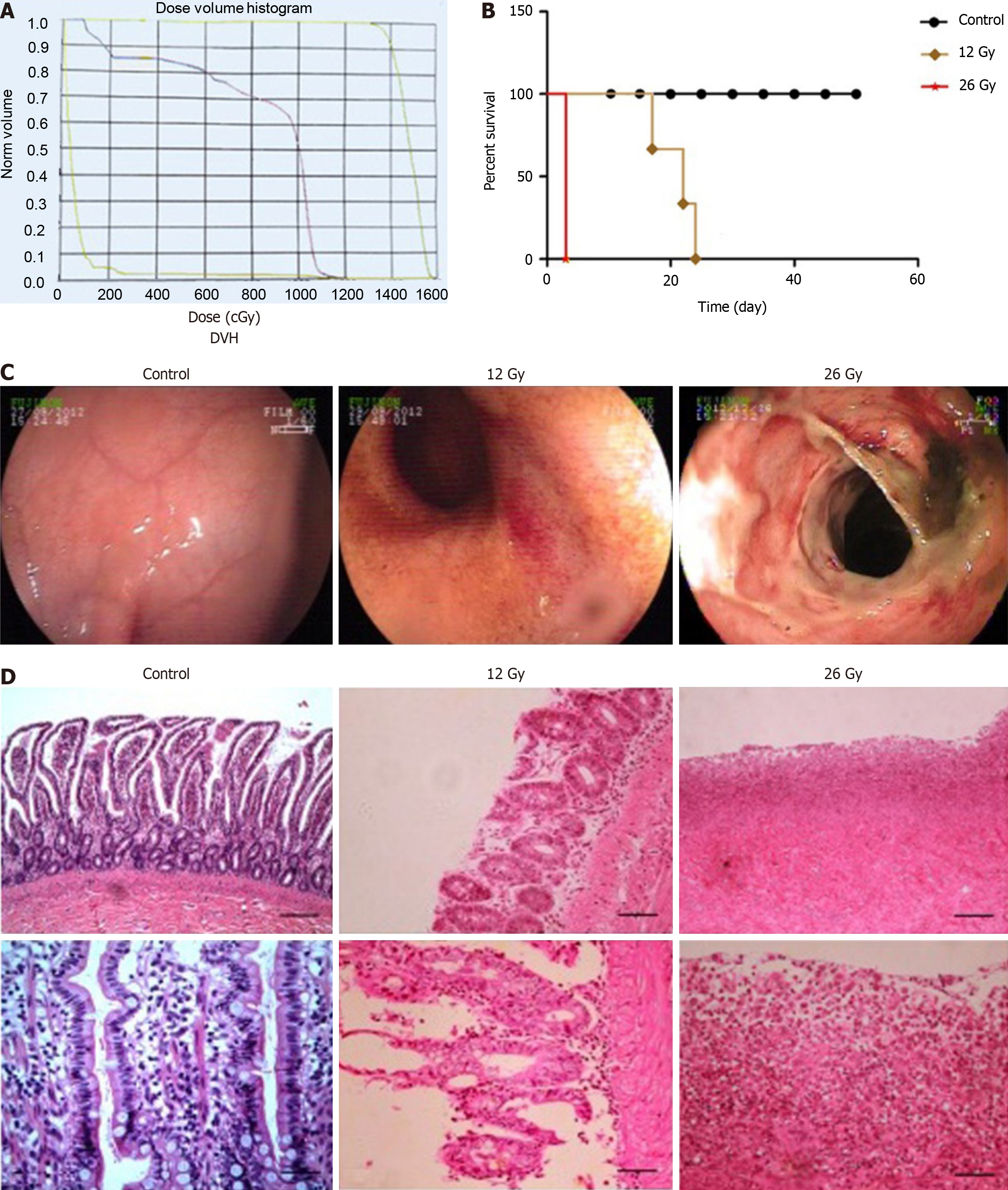
Dose and volume histogram made certain that 99% of the planning volume was covered by 95% of target dosage (Figure 2A). After irradiation, the temperature of dogs treated without irradiation changed within 1 °C, but animals treated with irradiation at a dose of 12 Gy exhibited temperature fluctuations within the range of 2-4 °C with the same supportive care. As for dogs treated with irradiation at a dose of 26 Gy, temperature increased significantly in 3 days (Table 1).
| Time | Control (n = 6) | 12 Gy (n = 6) | 26 Gy (n = 6) | |||
| T (°C) | W (kg) | T (°C) | W (kg) | T (°C) | W (kg) | |
| Day 1 | 38.73 ± 0.54 | 11.86 ± 0.42 | 38.67 ± 0.47 | 12.10 ± 0.53 | 38.93 ± 0.6 | 11.80 ± 0.53 |
| Day 3 | 38.73 ± 0.41 | 11.89 ± 0.42 | 39.30 ± 0.44 | 11.97 ± 0.59 | 41.63 ± 0.06a | 10.23 ± 0.90a |
| Day 5 | 38.75 ± 0.57 | 11.91 ± 0.38 | 39.73 ± 0.12 | 11.40 ± 0.87 | N | N |
| Day 7 | 38.70 ± 0.49 | 11.96 ± 0.39 | 40.23 ± 0.35a | 10.47 ± 0.75a | N | N |
| Day 9 | 38.71 ± 0.39 | 12.03 ± 0.43 | 40.77 ± 0.25a | 10.33 ± 0.51a | N | N |
| Day 11 | 38.76 ± 0.52 | 12.04 ± 0.41 | 40.30 ± 0.66a | 10.73 ± 0.38a | N | N |
The changes in weight were related to the treatment doses, and as the radiation dose increased, there was a declining trend in weight at each detection time point (Table 1). Weight loss appeared much earlier at high irradiation doses, compared with the control group. The group treated with 26 Gy exhibited apparent weight loss on day 3 after irradiation, whereas weight loss in the 12 Gy group appeared on day 7.
Early toxicity and mortality were dependent upon the single dose. High-dose abdominal irradiation was linked to severe intestinal toxicity and increased mortality (Figure 2B).
The endoscopic images were correlated with the irradiation doses on the first day after irradiation (Figure 2C). This normal mucosa was smooth, and the mucosal inflammation was severe and exhibited multifocal congestion, edema, and petechial hemorrhage at the dose of 12 Gy. Typical ulcers were observed with 26 Gy of irradiation. The visible injury included extensive mucosal exfoliation and loss of vascular architecture after irradiation.
With 0 Gy of radiation, samples taken from the small intestine did not exhibit any changes on day 3 post-irradiation. Moderate mucosal damage was detected in dogs treated with 12 Gy. These animals exhibited a partially damaged mucosa, glandular dilatation, inflammatory infiltration, vascular congestion, and hemorrhage. The mucosal damage was worse for dogs administered at 26 Gy, with severe histologic findings, including diffuse intestinal necrosis, erosions, and exfoliation (Figure 2D).
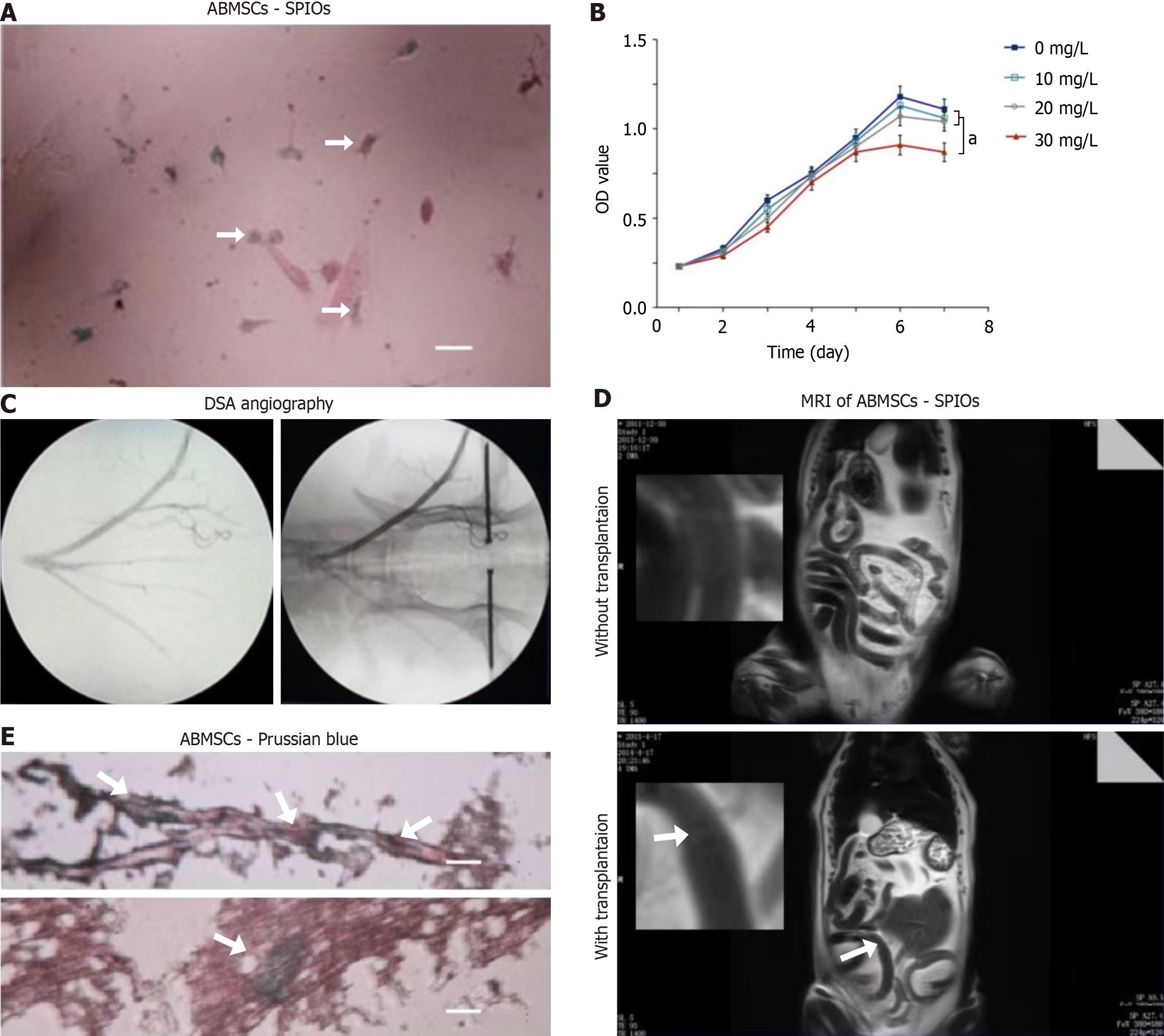
Transmission electronic microscopy revealed many vesicle-like inclusions containing small SPIO particles distributed in the cytoplasm (Figure 3A). MTT assay showed that ABMSC viability was not significantly affected when SPIO concentration was less than 20 mg/L (P > 0.05), but was significantly inhibited when SPIO concentration was 30 mg/L (P < 0.05; Figure 3B).
The SPIO-labeled ABMSCs were then transplanted into the bowel mesenteric artery to access the blood supply of the site of radiation-induced injury (Figure 3C). The MRI showed the results of T2 weighted image intensity comparison at the same layer. After transplantation of SPIO-labeled ABMSCs for 2 days, SPIO-marked ABMSCs were observed (Figure 3D, white arrow). Examination of frozen sections of intestinal tissue under a light microscope revealed that tissue sections stained with SPIOs showed blue granular intracellular clusters (Figure 3E).
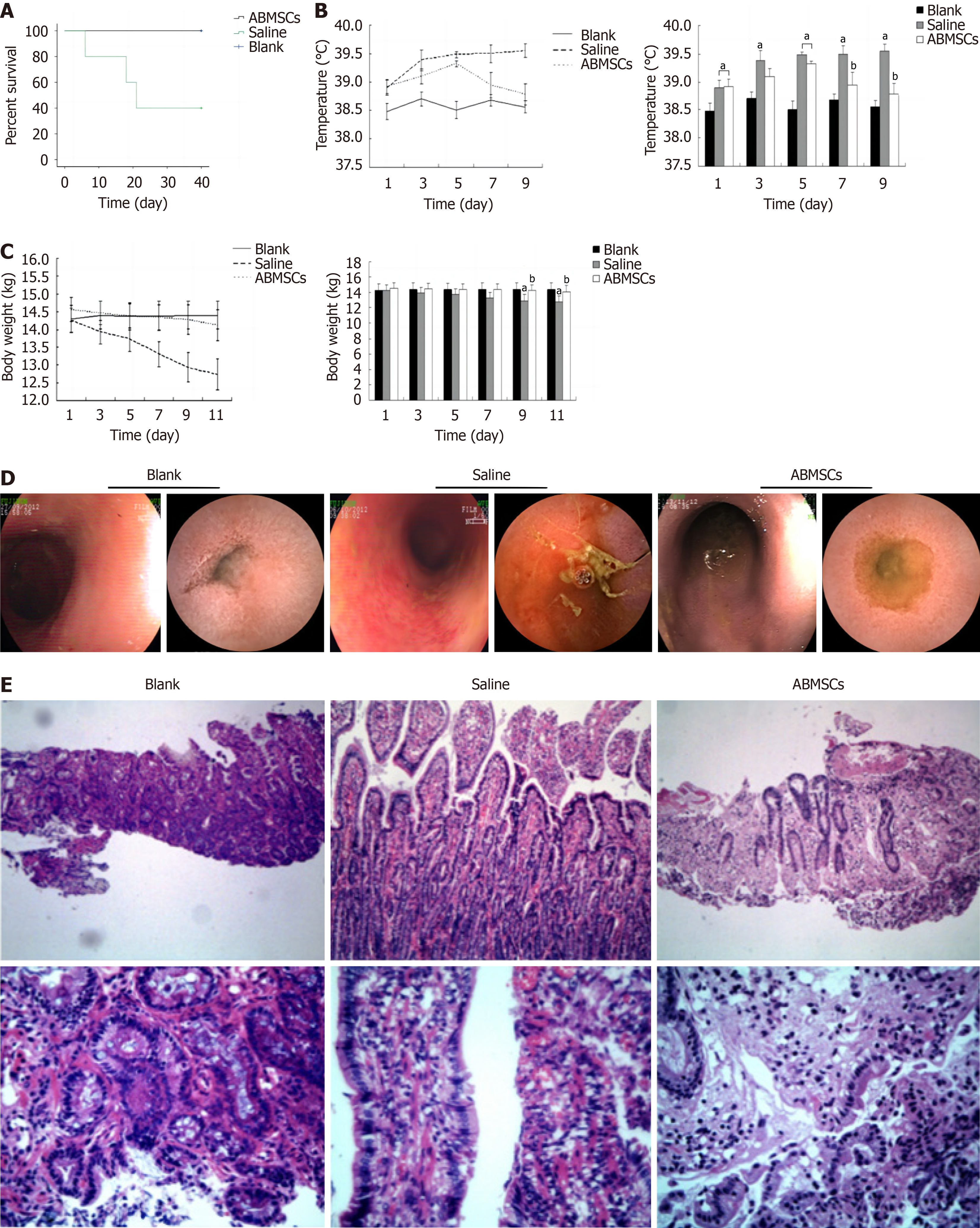
Three dogs died in 40 days in the saline treatment group while the survival rate in the ABMSCs treatment group was 100%. The survival rate was significantly higher in the ABMSCs treatment group than in the saline treatment group (P = 0.049; Figure 4A). The body temperature was higher in the saline treatment group than in the blank control group after irradiation (P < 0.05), and we also observed lower temperature in the ABMSCs treatment group than in the saline treatment group 5 days post treatment (P < 0.05; Figure 4B). Less obvious weight loss was observed in the ABMSCs treatment group than in the saline treatment group 7 days post treatment (Figure 4C). After treatment, dogs in the ABMSCs treatment group showed a lower nausea or vomiting frequency than those of the saline treatment group according to the daily record. Compared to animals in the saline treatment group, those of the ABMSCs treatment group exhibited relatively less diarrhea or bloody stools, and more food or water intake from observation after treatment.
Seven days post treatment, we found that intestinal damage improved more obviously in the ABMSCs treatment group than in the saline treatment group (Figure 4D). After intestinal irradiation, hematoxylin and eosin staining showed moderate mucosa damage, glandular dilatation, and severe inflammatory infiltration. In the ABMSCs treatment group, we observed recovered mucosal integrity, less vascular congestion, and relieved inflammatory infiltration (Figure 4E). According to histologic score (Table 2), inflammatory infiltration score was significantly lower after treatment than before (P = 0.003) and total histologic score was also significantly lower after treatment than before (P = 0.005) in the ABMSCs treatment group. However, there was no statistical difference in total histologic score before and after treatment in the saline treatment group (P > 0.05).
| Group | Time | Mucosa damage | Inflammatory infiltration | Vascular congestion | Total score |
| Blank | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Saline | Before transplantation | 2.6 ± 0.5 | 2.6 ± 0.5 | 1.6 ± 0.5 | 6.8 ± 1.6 |
| Saline | After transplantation | 2.2 ± 0.4 | 1.8 ± 0.8 | 1.4 ± 0.5 | 5.2 ± 1.6 |
| ABMSCs | Before transplantation | 2.4 ± 0.5 | 2.8 ± 0.4 | 1.4 ± 0.5 | 6.6 ± 1.3 |
| ABMSCs | After transplantation | 1.6 ± 0.5 | 1.2 ± 0.4a | 1.0 ± 0.0 | 3.8 ± 0.8a |
Quantibody® Canine Cytokine Array analysis was applied to detect the cytokines IL-2, IL-6, IL-8, IL-10, GM-CSF, MCP-1, RAGE, SCF, TNF-α, VEGF, erythropoietin, FGF-7, HGF, HGFR, IFN-γ, IL-1β, IL-12p40, IL-17A, MIP-1β, and TNF RI in intestinal samples. IL-10 and MCP-1 in intestinal samples which were collected 7 days post treatment showed significant differences between the ABMSCs treatment group and saline treatment group (P = 0.027 and P = 0.039, respectively; Figure 5A). The cytokines IL-10 and MCP-1 in the intestine were elevated significantly after ABMSCs treatment for 7 days (P < 0.05; Figure 5B and C). The ELISA results showed that the serum concentrations of IL-10 and MCP-1 in the ABMSCs treatment group were 2439.20 ± 63.03 pg/mL and 1988.20 ± 80.41 pg/mL, respectively, and they were 207.41 ± 26.85 pg/mL and 266.53 ± 11.77 pg/mL, respectively, in the saline treatment group; there were significant differences between the two groups (P < 0.05; Figure 5D).
Currently, human MSCs are commonly isolated from various tissues using explant and enzymatic protocols[8]. There are several MSC types, such as adipose-derived MSCs, umbilical-cord-derived MSCs, tonsil-derived MSCs, dental-pulp-derived MSCs, and bone-marrow-derived MSCs. Human bone-marrow-derived MSCs (hBM-MSCs) are attractive radioactive enteritis therapeutic agents owing to their widespread availability, easy attainability and in vitro manipulation, and the lack of ethical issues. Ex vivo hBM-MSC expansion before transplantation is essential because of the low cell numbers in bone marrow aspirates, and it is worth noting that 3D culture, dynamic conditions, and physiological oxygen levels are critical aspects in this process to maintain and even increase the therapeutically relevant properties of MSCs[9].
Bone marrow was thought to be a potential source to reconstitute the human gastrointestinal epithelium as emergent tissue recovery was needed[10]. MSCs’ therapeutic effects on intestinal damage have been reported in some studies[11,12]. Transplantation of MSCs improved radiation-induced intestinal injury and could engraft into the enteric mucosa[13]. However, the exact mechanisms of MSC application on radioactive enteritis are not totally clear. In nonhuman primates models, researchers reported that transplanted MSCs were distributed in intestinal tissues after irradiation and were involved in the repairing process[14,15]. MSCs had the ability of re-establishing cellular homeostasis, accelerating structure repair, and promoting functional recovery. Functions of secretory and absorptive response of radioactive intestinal epithelium were improved by MSCs which are capable of restoring intestine integrity and regulating epithelial cells[1,16]. It has been concluded that intestinal crypt villus has the ability of self-renewing and a single intestinal stem cell can work independently, expand continuously, and regulate the epithelium to the normal status[17]. In the present study, MSCs were cultured from bone marrow of dogs and were identified with monoclonal antibodies. After radiation, the survival rate in the ABMSCs treatment group was higher than that of the saline treatment group. Histological analysis from endoscopy examination proved the efficacy of MSC transplantation in radioactive enteritis and the result was similar to those of previous studies[2-4].
MSCs that are adherent cells have the ability of self-renewal as well as multilineage differentiation[18]. However, current research suggests that the therapeutic effects of MSCs in many diseases are attributed to their paracrine-mediated actions or bystander effects, rather than to cell replenishment or replacement in defective and necrotic areas through cell proliferation and differentiation[19-22]. This therapeutic plasticity is due to MSCs’ ability to secrete a broad range of bioactive molecules including growth factors, trophic factors, cytokines, chemokines, and extracellular vesicles. Inflammatory chemokines were reported to improve survival by limiting inflammatory response as well as radioactive damage in mice following MSC transplantation[23]. MSCs had the ability of secreting various cytokines such as SCF, IL-3, megakaryocyte growth and development factor, and FLT-3 Ligand[24]. Researchers also found that some pro-inflammatory cytokines such as IFN-γ, IL-8, TGF-α, and VCAM-1 could modulate MSCs with regard to their paracrine, trafficking, and immunosuppressing potential[25-27]. After bone marrow stromal cell transplantation in mice with radiation-induced gastrointestinal syndrome, KGF, PDGF, FGF2, and anti-inflammatory cytokines in serum were reported to be up-regulated while inflammatory cytokines decreased[3]. In this study, we found that the level of the anti-inflammatory cytokine IL-10 in serum and intestine tissue rapidly increased after ABMSC transplantation compared with the control groups. This result suggests that ABMSC transplantation may increase the levels of anti-inflammatory cytokines and inhibit inflammatory cytokines to reduce the risk of systemic inflammatory response syndrome, thereby contributing to the repair of intestinal radiation injury. Our conclusion is similar to that reported by Chang et al[28]. At the same time, we found that the secretion of MCP-1 in intestinal tissues increased dramatically after AMSC transplantation, which was similar to previous studies[6]. This result may explain the possible mechanism of MSCs’ participation in the repair of radioactive enteritis. In fact, in this research, we first examined MCP-1 at 7 days post ABMSC treatment in dog models. The possible reasons may involve that we chose this time point to facilitate endoscopy observation and tissue collection. Previous studies mostly reported changes of other inflammatory cytokines and growth factors in rodent animals while Beagle dog model was applied in this study.
In brief, transplantation of ABMSCs proved efficacious and was capable of promoting intestinal recovery from acute radioactive injury in Beagle dogs. It may provide a new therapeutic method for radiation-induced enteritis. IL-10 and MCP-1 may play important roles in ABMSC treatment and the exact mechanism needs to be explored. Although this work confirms that cell therapy using ABMSCs could be a functional approach to treat acute radioactive enteritis in Beagle dogs, further investigation employing more in-depth mechanistic studies is required before making any clinical conclusion for humans, which can facilitate the MSC modification and enhance their future clinical use. Furthermore, a large-scale process for the manufacturing of healthy and potent cells and cellular products under continuous physiologic conditions is still missing. In the future, more efforts are necessary to combine the advances in 3D culture, especially in the field of hydrogels, monitoring platforms, and bioreactor technology to generate a well-defined, physiologic environment for the isolation and expansion of MSCs[29]. And in clinical applications, the quality of hBM-MSCs should be assessed after cell detachment from the culture dish, because cell quality is an essential factor for therapeutic effects. However, there are limitations to the traditional methods used to evaluate the quality and freshness of cells prior to transplantation of hBM-MSCs into humans[30]. Our future research will focus on the above points.
| 1. | Sémont A, Mouiseddine M, François A, Demarquay C, Mathieu N, Chapel A, Saché A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Kudo K, Liu Y, Takahashi K, Tarusawa K, Osanai M, Hu DL, Kashiwakura I, Kijima H, Nakane A. Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J Radiat Res. 2010;51:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6:e24072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Xu W, Chen J, Liu X, Li H, Qi X, Guo X. Autologous bone marrow stromal cell transplantation as a treatment for acute radiation enteritis induced by a moderate dose of radiation in dogs. Transl Res. 2016;171:38-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 6. | Lee SH, Jin KS, Bang OY, Kim BJ, Park SJ, Lee NH, Yoo KH, Koo HH, Sung KW. Differential Migration of Mesenchymal Stem Cells to Ischemic Regions after Middle Cerebral Artery Occlusion in Rats. PLoS One. 2015;10:e0134920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Goldner G, Tomicek B, Becker G, Geinitz H, Wachter S, Zimmermann F, Wachter-Gerstner N, Reibenwein J, Glocker S, Bamberg M, Feldmann H, Pötzi R, Molls M, Pötter R. Proctitis after external-beam radiotherapy for prostate cancer classified by Vienna Rectoscopy Score and correlated with EORTC/RTOG score for late rectal toxicity: results of a prospective multicenter study of 166 patients. Int J Radiat Oncol Biol Phys. 2007;67:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Lyu Z, Xin M, Oyston DR, Xue T, Kang H, Wang X, Wang Z, Li Q. Cause and consequence of heterogeneity in human mesenchymal stem cells: Challenges in clinical application. Pathol Res Pract. 2024;260:155354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 9. | Kim SG, George NP, Hwang JS, Park S, Kim MO, Lee SH, Lee G. Human Bone Marrow-Derived Mesenchymal Stem Cell Applications in Neurodegenerative Disease Treatment and Integrated Omics Analysis for Successful Stem Cell Therapy. Bioengineering (Basel). 2023;10:621. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Lange C, Brunswig-Spickenheier B, Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C, Schlegelberger B, Cornils K, Zustin J, Spiess AN, Zander AR. Radiation rescue: mesenchymal stromal cells protect from lethal irradiation. PLoS One. 2011;6:e14486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Zhang J, Gong JF, Zhang W, Zhu WM, Li JS. Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci. 2008;15:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E, Trompier F, Dudoignon N, Germain C, Mazurier C, Aigueperse J, Borneman J, Gorin NC, Gourmelon P, Thierry D. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med. 2003;5:1028-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 323] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 554] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 16. | Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4394] [Cited by in RCA: 5173] [Article Influence: 323.3] [Reference Citation Analysis (0)] |
| 18. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 19. | Huang X, Yang X, Huang J, Wei L, Mao Y, Li C, Zhang Y, Chen Q, Wu S, Xie L, Sun C, Zhang W, Wang J. Human amnion mesenchymal stem cells promote endometrial repair via paracrine, preferentially than transdifferentiation. Cell Commun Signal. 2024;22:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 20. | Miao J, Ren Z, Zhong Z, Yan L, Xia X, Wang J, Yang J. Mesenchymal Stem Cells: Potential Therapeutic Prospect of Paracrine Pathways in Neonatal Infection. J Interferon Cytokine Res. 2021;41:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Zhang X, Liu T, Ran C, Wang W, Piao F, Yang J, Tian S, Li L, Zhao D. Immunoregulatory paracrine effect of mesenchymal stem cells and mechanism in the treatment of osteoarthritis. Front Cell Dev Biol. 2024;12:1411507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 22. | Qi L, Hu L, Qian R, Ye B, Feng Y, Deng Y, Wang C, Zhou C, Liu G, Gao X, Lin C, Ding Q, Song C, Zhao Z, Lin Z, Zhu J, Zhang M. Advances in mesenchymal stem cell-centered stem cell therapy in the treatment of hypoxic-ischemic injury. Int Immunopharmacol. 2024;143:113430. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Van der Meeren A, Mouthon MA, Vandamme M, Squiban C, Aigueperse J. Combinations of cytokines promote survival of mice and limit acute radiation damage in concert with amelioration of vascular damage. Radiat Res. 2004;161:549-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, Thullier P, Lataillade JJ, Herodin F. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood. 2004;103:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 26. | Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Hou Y, Ryu CH, Jun JA, Kim SM, Jeong CH, Jeun SS. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014;38:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H, Jin X. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis. 2013;4:e685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Egger D, Lavrentieva A, Kugelmeier P, Kasper C. Physiologic isolation and expansion of human mesenchymal stem/stromal cells for manufacturing of cell-based therapy products. Eng Life Sci. 2022;22:361-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Lee DY, Lee SE, Kwon DH, Nithiyanandam S, Lee MH, Hwang JS, Basith S, Ahn JH, Shin TH, Lee G. Strategies to Improve the Quality and Freshness of Human Bone Marrow-Derived Mesenchymal Stem Cells for Neurological Diseases. Stem Cells Int. 2021;2021:8444599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |