Published online Feb 21, 2025. doi: 10.3748/wjg.v31.i7.96459
Revised: December 1, 2024
Accepted: December 27, 2024
Published online: February 21, 2025
Processing time: 257 Days and 19 Hours
Excessive endoplasmic reticulum (ER) stress in intestinal epithelial cells can lead to damage to the intestinal mucosal barrier, activate the signal transducer and activator of transcription 3 (STAT3)/nuclear factor kappa B (NF-κB) signaling pathway, and exacerbate the inflammatory response, thus participating in the pathogenesis of ulcerative colitis (UC). Mesalazine is a commonly used drug in the clinical treatment of UC. However, further studies are needed to determine whether mesalazine regulates the ER stress of intestinal epithelial cells, down-regulates the STAT3/NF-κB pathway to play a role in the treatment of UC.
To study the therapeutic effects of mesalazine on spontaneous colitis in inter
The 24-week-old IL-10-/- mice with spontaneous colitis were divided into the model group and the 5-amino salicylic acid group. Littermates of wild-type mice of the same age group served as the control. There were eight mice in each group, four males and four females. The severity of symptoms of spontaneous colitis in IL-10-/- mice was assessed using disease activity index scores. On day 15, the mice were sacrificed. The colon length was measured, and the histopathological changes and ultrastructure of colonic epithelial cells were detected. The protein expressions of STAT3, p-STAT3, NF-κB, IκB, p-IκB, and glucose-regulated protein 78 were identified using Western blotting. The STAT3 and NF-κB mRNA expressions were identified using real-time polymerase chain reaction. The glucose-regulated protein 78 and C/EBP homologous protein expressions in colon sections were detected using immunofluorescence.
Mesalazine reduced the symptoms of spontaneous colitis in IL-10 knockout mice and the histopathological damage of colonic tissues, and alleviated the ER stress in epithelial cells of colitis mice. Western blotting and quantitative real-time polymerase chain reaction results showed that the STAT3/NF-κB pathway in the colon tissue of model mice was activated, suggesting that this pathway was involved in the pathogenesis of UC and might become a potential therapeutic target. Mesalazine could down-regulate the protein expressions of p-STAT3, NF-κB and p-IκB, and down-regulate the mRNA expression of STAT3 and NF-κB.
Mesalazine may play a protective role in UC by reducing ER stress by regulating the STAT3/NF-κB signaling pathway.
Core Tip: Long-term and persistent excessive endoplasmic reticulum stress in IL-10-/- mice intestinal epithelial cells destroy the intestinal mucosal barrier and activates the signal transducer and activator of transcription 3/nuclear factor kappa B pathway, leading to the spontaneous generation of ulcerative colitis. Mesalazine could alleviate the symptoms of colitis in IL-10-/- mice, regulate endoplasmic reticulum stress and down-regulate the signal transducer and activator of transcription 3/nuclear factor kappa B pathway.
- Citation: Chen Q, Zhang YL, Shi YQ, Zheng L. Mesalazine alleviated the symptoms of spontaneous colitis in interleukin-10 knockout mice by regulating the STAT3/NF-κB signaling pathway. World J Gastroenterol 2025; 31(7): 96459
- URL: https://www.wjgnet.com/1007-9327/full/v31/i7/96459.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i7.96459
Inflammatory bowel disease (IBD) is a chronic disease and is divided into Crohn’s disease and ulcerative colitis (UC). Typically characterized by chronic diarrhea (with or without bleeding), abdominal pain, and weight loss. Diagnosis is based on history, physical examination, laboratory studies, and endoscopic evaluation[1,2]. According to several studies, the prevalence of UC is still high and tends to be stable in the western world. However, the prevalence of UC is rapidly increasing in the newly industrialized nations, and UC is becoming a global disease[3]. The pathogenesis of UC has not been extensively explored, which may be associated with gene-environment interaction, diet, intestinal barrier des
Endoplasmic reticulum (ER) stress is a common stress response that can induce cell apoptosis and disrupt cell homeostasis. Numerous studies have demonstrated that excessive ER stress impairs the function of the intestinal mucosal barrier and contributes to the occurrence of IBD[7-10]. To maintain intestinal homeostasis, intestinal epithelial cells, including mucus-secreting goblet cells and antimicrobial peptide-producing Paneth cells, secrete a significant amount of proteins and are more susceptible to ER stress[11]. Glucose-regulated protein 78 (GRP78) is a member of the heat shock protein 70 family and exists in the ER membrane of eukaryotes[12]. Under steady-state conditions, GRP78, in its inactive form, binds to the pancreatic ER eIF2α kinase, inositol-requiring enzyme 1, and activating transcription factor 6. Moreover, under ER stress, GRP78 activates inositol-requiring enzyme 1, pancreatic ER eIF2α kinase, or activating transcription factor 6 and initiates the unfolded protein response, promoting the correct folding of the protein and inhibiting protein synthesis[13]. Therefore, GRP78 can be considered a marker protein for ER stress. The expression of GRP78 increases under ER stress. Longer persistence of ER stress may activate C/EBP homologous protein (CHOP) and induce apoptosis[14].
Mesalazine [5-amino salicylic acid (5-ASA)] is the main therapeutic agent for IBD in the mild active stage, which can significantly inhibits the inflammation of intestinal mucosa[15]. The mechanisms by which 5-ASA alleviates the symptoms of UC include inhibition of cyclooxygenase, peroxisome proliferator-activated receptor γ, nuclear factor kappa B (NF-κB), and immunosuppressive effects[16]. However, the regulatory effect on ER stress is not clear. This study aimed to determine whether GRP78, a marker protein for ER stress, can become a potential therapeutic target of IBD by mediating inflammatory pathways. Therefore, we hypothesized that 5-ASA might treat colitis by regulating the unfolded protein response in colonic epithelial cells. This study aimed to assess whether 5-ASA could reduce the severity of colitis in interleukin-10 (IL-10)-/- mice by regulating ER stress.
The 24-week-old IL-10-/- wild-type mice under C57BL/6 background were raised in a specific pathogen free environment in the Animal Laboratory Center of Shanghai University of Traditional Chinese Medicine [License No. SCXK (Shanghai) 2020-0009]. According to the standard cycle of 12 hours of light/12 hours of darkness, mice were kept at room temperature (24 ± 2 °C) and relative humidity between 50%-60%. The genetic testing services were provided by the Shanghai Model Organisms Center, Inc. This study was approved by the Laboratory Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (Ethics No. PZSHUTCM191108004).
The 24-week-old IL-10-/- spontaneous enteritis mice were divided into the IL-10-/- group and the 5-ASA group. The same-week-old littermates were considered the control group. There were eight mice in each group, four males and four females. Mesalazine enteric-coated tablets (Huidi), purchased from Sunflower Pharmaceutical Group Co., ltd. The 5-ASA group was treated by gavage (100 mg/kg/day) for two weeks, while the wild group and the IL-10-/- group were given saline for two weeks.
During the experiment, the rectal prolapse length of each mouse was measured, their stool traits were observed, and the fecal occult blood was measured and recorded. Disease activity index scoring was conducted based on the method of Zuo et al[17]. One point was scored for each characteristic, including wrinkled hair, occult blood, rectal prolapse < 1 mm and soft stool, and diarrhea or severe rectal prolapse > 1 mm.
The colon tissue fixed in 10% neutral formalin buffer was dehydrated and embedded in paraffin. Sections of the colon were stained with hematoxylin and eosin. The pathological changes were observed under an optical microscope.
Fresh tissue from the terminal colon was cut into 1 mm3 section and fixed in 2.5% glutaraldehyde and 1% osmic acid. Colon epithelial cells were dehydrated with gradient acetone, embedded, and allowed to cure for 48 hours. The cells were sectioned into a thickness of 50-60 nm and double stained with uranium acetate and lead citrate. The ultrastructure of ER of colon epithelial cells was observed under a transmission electron microscope, and the pictures were captured.
The protein expression levels of activator of transcription 3 (STAT3), p-STAT3, NF-κB, IκB, p-IκB, and GRP78 in the colon tissue were determined. The total protein was extracted from the colon of mice, and the protein concentration was determined using the bicinchoninic acid method. The same amount of proteins was separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to the polyvinylidene fluoride membrane. After blocking 5% skim milk for 1 hour at room temperature, polyvinylidene fluoride membrane was further incubated with primary antibody at 4 °C overnight. Using primary antibodies against the following proteins: GRP78 (1:2000, 11587-1-AP, Proteintech), STAT3 (1:1000, A19566, ABclonal), p-STAT3(1:500, AP0715, ABclonal), NF-κB (1:1000, 8242S, Cell Signaling Technology), IκB (1:1000, 9242S, Cell Signaling Technology), p-IκB (1:1000, 2859S, Cell Signaling Technology) and β-actin (1:1000, 4970S, Cell Signaling Technology). The next day, the membrane was incubated with the anti-rabbit IgG (1:3000, 7074S, Cell Signaling Technology) at room temperature for 1 hour. The enhanced chemiluminescence detection kit was used to determine the exposure after thrice washing with Tris-buffered saline with Tween 20. β-actin was set as the internal parameter, and Image J software was used to analyze the obtained luminescence signals.
Total RNA was extracted from colon tissues using Trizol, and its concentration was determined. The RNA was reverse-transcribed into cDNA using a reverse transcription kit. The ABI StepOnePlus real-time fluorescence quantitative polymerase chain reaction (PCR) instrument was used to carry out the PCR amplification and detection. The real-time PCR reaction consisted of precycling at 95 ˚C for 30 seconds, denaturation at 95 ˚C for 5 seconds, and annealing at 60 ˚C for 30 seconds for 40 cycles. The melting curve was monitored to confirm the specificity of the PCR products. The mRNA expression level of the target gene relative to the reference gene β-actin was determined by 2-△△Ct method. The primers were designed and synthesized by Shanghai Shinegene Biotechnology Co., ltd. (Tables 1 and 2).
| 0 | 1 | 2 | |
| Ruffled fur | - | + | / |
| Occult fecal blood | - | + | / |
| Rectal prolapse | - | < 1 mm | > 1 mm |
| Stool consistency | - | Soft stool | Diarrhea |
| Gene name | Forward primer | Reverse primer |
| STAT3 | CTGGCTAGACAATATCATCGACC | GGCTTTGTGCTTAGGATGGC |
| NF-κB | GTGGAGGCATGTTCGGTAGTG | TCTTGGCACAATCTTTAGGGC |
| β-actin | GAGACCTTCAACACCCCAGC | TGCCTTACGGACCTCTTCTATC |
The 5 μm thick colon paraffin sections were deparaffinized with xylene, hydrated with a gradient of ethanol, immersed in antigen repair solution (citric acid buffer, 10 mmol/L, pH: 6, 98 °C, 15 minutes), and blocked for non-specific binding with normal goat serum for 30 minutes at room temperature. They were incubated overnight at 4 °C with rabbit anti-GRP78 polyclonal antibodies (1:100, 11587-1-AP, Proteintech) and rabbit anti-CHOP antibodies (1:100, 5554S, Cell Signaling Technology). The sections were washed thrice with phosphate buffer saline (PBS), incubated with different fluorescently labeled secondary antibodies (fluorescein isothiocyanate goat anti-rabbit IgG, 1:100, BA1105, Boster) at room temperature for 1 hour, and re-washed with PBS for 3 × 5 minutes. The sections were counterstained with 4’,6-diamidino-2-phenylindole for 5 minutes, washed with PBS for 3 × 5 minutes, and sealed with a cover tablet containing an anti-fluorescence quencher. The results were observed and photographed under a laser-scanning confocal microscope.
All data were expressed as mean ± SD. Use Shapiro-Wilk test to judge whether the data conforms to normal distribution. According to the normal distribution of variables, the differences between groups were analyzed by one-way analysis of variance or nonparametric Kruskal-Wallis test. SPSS 25.0 software was used for data analysis.
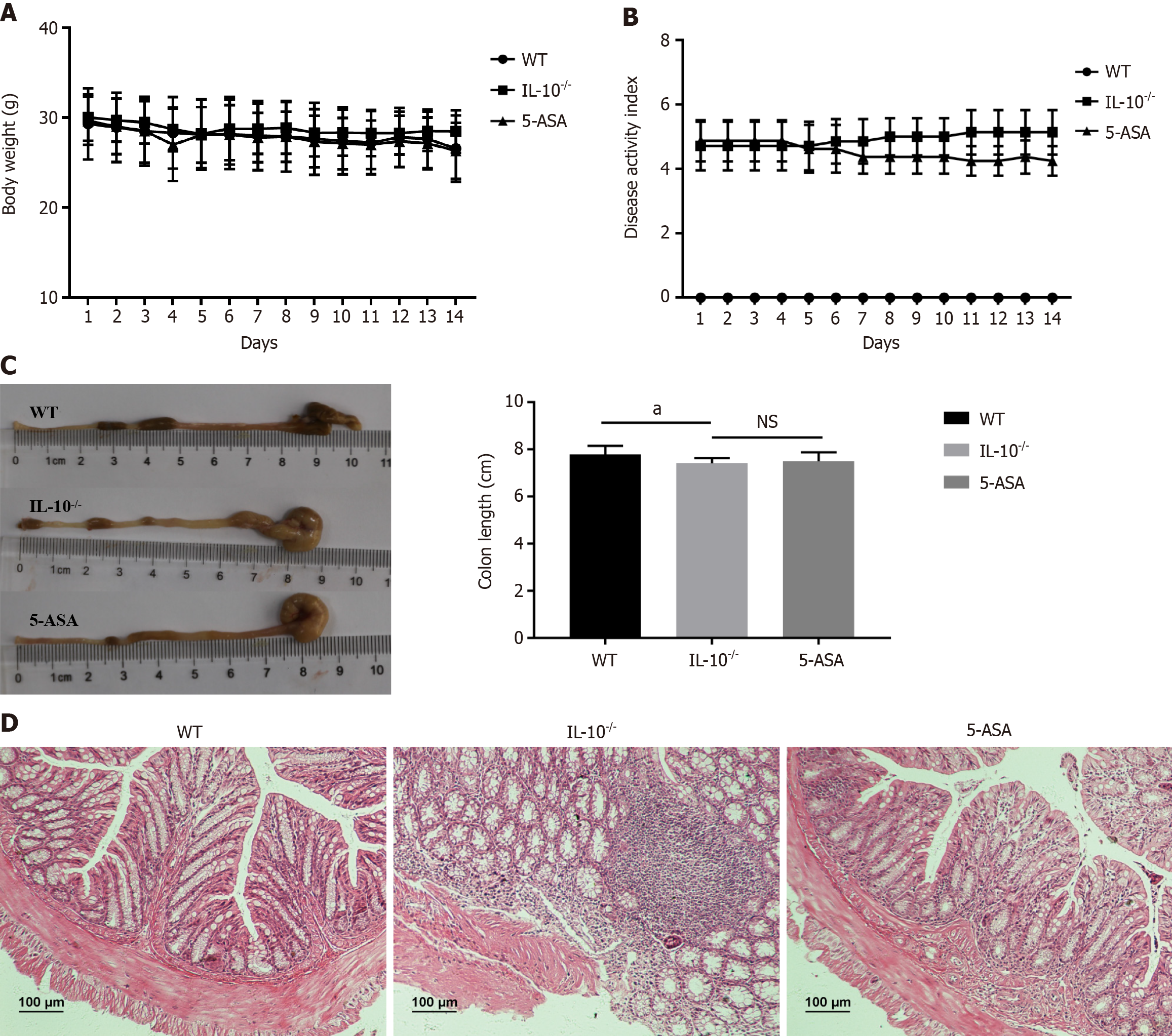
Rectal prolapse, fecal occult blood, and soft stools were not observed in the wild group. Mesalazine showed no significant effect on body weight and disease activity index score in IL-10-/- mice. The intestinal mucosa was intact, the glands were arranged neatly, and no ulcer or inflammatory cell infiltration was observed. The mucosa of IL-10-/- mice was exfoliated or necrotic, a significant number of inflammatory cells were infiltrated in the mucosa and submucosa, and some large ulcers were formed. Contrastingly, the inflammatory cell infiltration of colon tissue in the 5-ASA group was significantly reduced, and the appearance of mucosal histology was significantly improved (Figure 1).
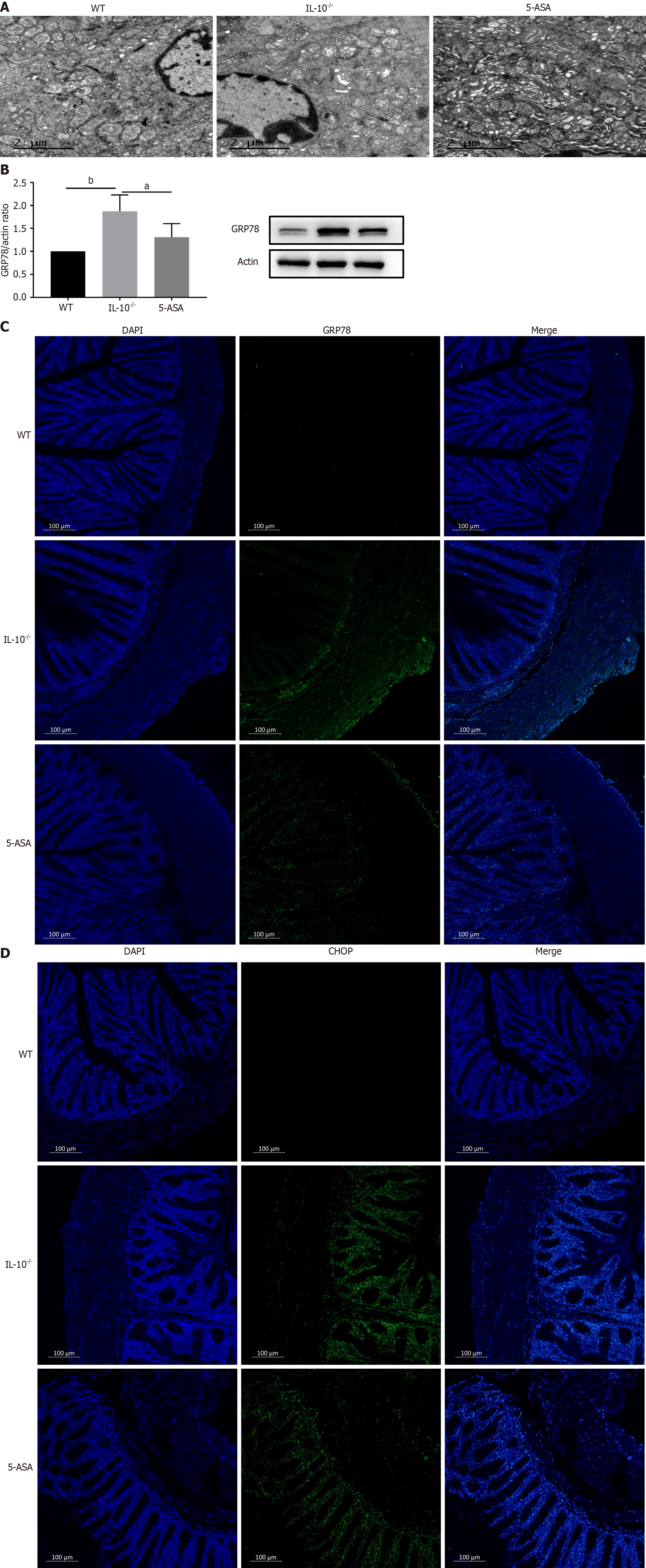
The ultrastructure of mouse colonic epithelial cells was observed under a transmission electron microscope. In the wild group, the structure of ER was clear, the omental lumen was not dilated, the organelles were normal, and a large number of ribosomes were found attached to it. The number of rough ER increased, and organelles swelled significantly in the IL-10-/- group. However, the number of rough ER was reduced in the 5-ASA group. The omental space of the ER was slightly dilated, some organelles were swollen, and the morphology was basically normal. Western blotting results showed that the expression of GRP78, a marker of ER stress, was significantly increased in the IL-10-/- group (P < 0.01) and decreased in the 5-ASA group (P < 0.05). Meanwhile, immunofluorescence results showed that the wild expressions of GRP78 and CHOP were significantly lower than those of other groups, and the expressions were significantly increased in the IL-10-/- group. To summarize, mesalazine can relieve ER stress of epithelial cells in colitis mice and significantly down-regulate GRP78 (Figure 2).
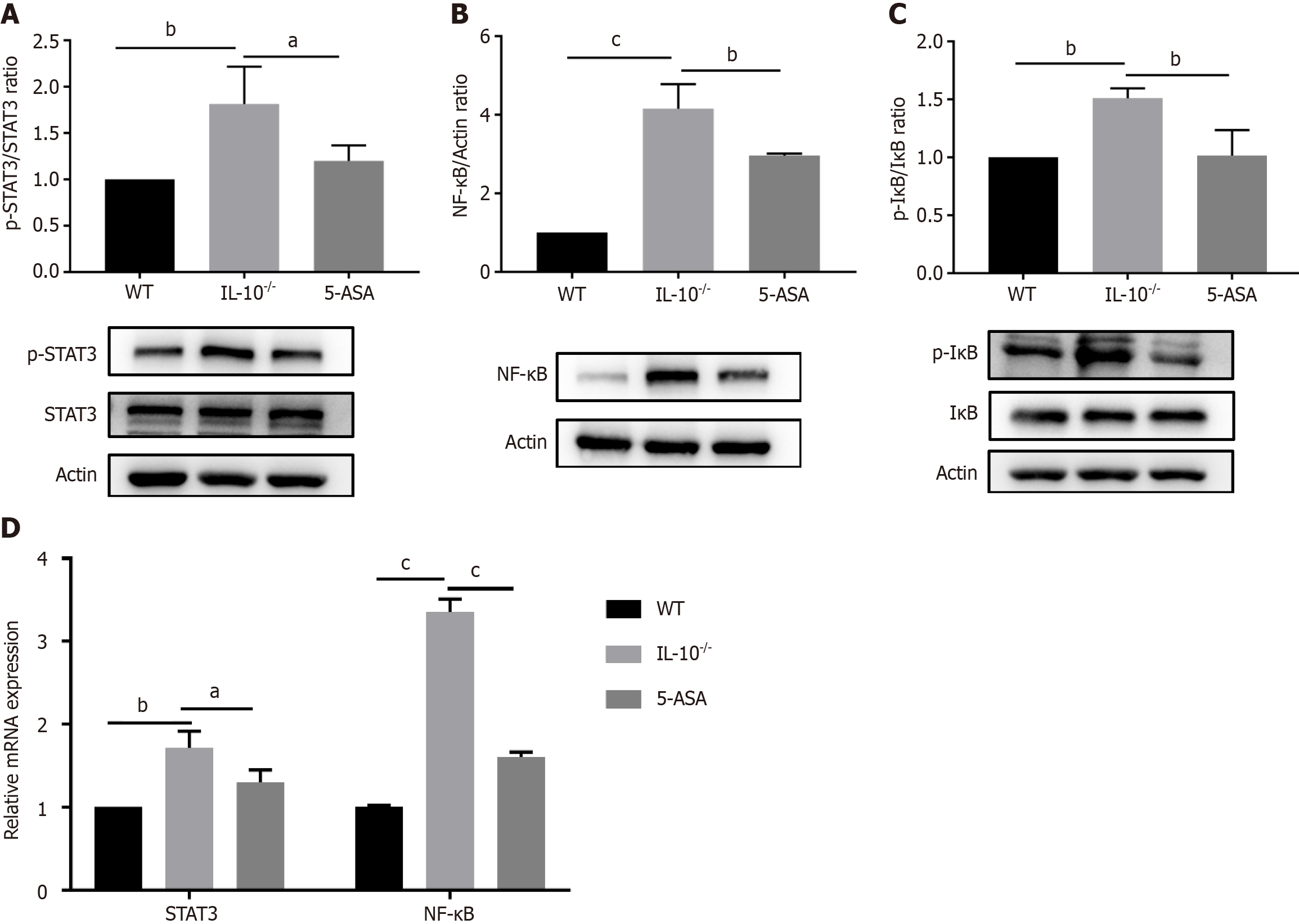
The anti-inflammatory mechanism of mesalazine was further explored by detecting genes and proteins associated with the STAT3/NF-κB signaling pathway using PCR and Western blot. As shown in the Figure 3, compared with the wild-type group, the protein expressions of p-STAT3 (P < 0.01), NF-κB (P < 0.001), and p-IκB (P < 0.01) in the IL-10-/- group were significantly increased, and the mRNA expressions of STAT3 (P < 0.01) and NF-κB (P < 0.001) were significantly increased, suggesting that the STAT3/NF-κB signaling pathway was activated in the colon tissue of IL-10-/- mice. Mesalazine significantly down-regulated protein and gene expression in the STAT3/NF-κB signaling pathway compared with the IL-10-/- group.
UC is a chronic IBD characterized by abdominal pain, diarrhea, mucopurulent and bloody stool. The incidence of UC is increasing worldwide. The most commonly prescribed drug today is mesalazine. Mesalazine has a lower incidence and severity of side effects than other therapies. However, its anti-inflammatory mechanism needs further investigation. The results showed that mesalazine treatment could effectively reduce the symptoms of colitis in mice, including weight loss, shortened colon length and tissue damage. IL-10-/- mice are common transgenic animal models to study the pathogenesis of IBD. IL-10-/- mice spontaneously develop chronic IBD within two to four months of birth and exhibit symptoms resembling those of human UC, such as infiltration of inflammatory cells into the lamina propria and submucosa, epithelial hyperplasia, mucin depletion, crypt abscesses, ulcers, and bowel wall thickening[18,19]. Therefore, the anti-inflammatory activity of Mesalazine was studied by using IL-10-/- mice model of spontaneous colitis.
STAT3 belongs to the family of signal transduction and transcription activation factors and is closely associated with the NF-κB signaling pathway[20]. STAT3 can be activated by IL-6, and when STAT3 is phosphorylated, it translocates to the nucleus and mediates the transcription of its target genes, causing inflammation-related tissue damage[21]. NF-κB is a nuclear transcription factor that often binds to and is inhibited by IκB. When IκB is phosphorylated by IκB kinase, IκB ubiquitinates and degrades to release NF-κB. The activated NF-κB is further phosphorylated and transferred to the nucleus to induce the transcription of inflammatory genes[22]. Studies have shown that the STAT3 and NF-κB signaling pathways play important roles in the disease progression of IBD[23-25]. Therefore, we examined whether mesalazine altered the activation of the STAT3/NF-κB pathway in this study. Mesalazine down-regulated the expression of STAT3/NF-κB pathway-related proteins and genes, which may be one of the mechanisms underlying the alleviation of spon
GRP78 is a marker protein of ER stress, and its expression is increased in excessive ER. In this study, the expression level of GRP78 protein in the IL-10-/- group was significantly higher than in the wild group. Electron microscopy results demonstrated that the colonic epithelial cells of IL-10-/- mice showed increased rough ER and obvious swelling of organelles, suggesting that ER stress occurred in the colon of IL-10-/- mice. Immunofluorescence results showed that the expression levels of GRP78 and CHOP in the IL-10-/- group were significantly higher than those in the wild-type group, while mesalazine could reduce ER stress and reduce the expression levels of GRP78 and CHOP. Upregulated GRP78 can be secreted into the extracellular as secreted GRP78 (sGRP78). Research has shown that GRP78 secreted into the extracellular has immune regulatory functions in inflammatory responses and is a molecular pattern related to inflammation resolution. The expression of sGRP78 is reduced in human colitis samples. During the active phase of dextran sulfate sodium salt-induced colitis, the sGRP78 levels significantly decrease, but rapidly rebound during resolving phase[26]. In future experiments, we should further detect the expression of sGRP78 in plasma and supernatant of colonic mucosal punches.
In conclusion, our study showed that there is significant ER stress in the colonic epithelial cells of IL-10-/- mice. Mesalazine can down-regulate GRP78 expression, inhibit ER stress and reduce inflammation. Based on the existing mechanism of mesalazine inhibiting inflammation, a potentially novel mechanism is added; mesalazine provides an effective treatment target for UC. However, there are still some limitations in our study. This experimental study has a short cycle. We found that mesalazine can reduce intestinal inflammation in IL-10-/- mice in the short term and regulate ER stress in the colonic epithelial cells of mice. However, the repair effect of mesalazine on the intestinal mucosal barrier still needs to be validated by further long-term experiments. In the future experiments, we will try to use molecular inhibitors or gene knockout/overexpression methods, expand the sample size, try different dosage forms of mesalazine and other experimental designs to further improve our experiments.
We would like to thank our colleagues in the Institute of Digestive Disease affiliated to Shanghai University of Traditional Chinese Medicine for their help and support in this research.
| 1. | Gordon H, Minozzi S, Kopylov U, Verstockt B, Chaparro M, Buskens C, Warusavitarne J, Agrawal M, Allocca M, Atreya R, Battat R, Bettenworth D, Bislenghi G, Brown SR, Burisch J, Casanova MJ, Czuber-Dochan W, de Groof J, El-Hussuna A, Ellul P, Fidalgo C, Fiorino G, Gisbert JP, Sabino JG, Hanzel J, Holubar S, Iacucci M, Iqbal N, Kapizioni C, Karmiris K, Kobayashi T, Kotze PG, Luglio G, Maaser C, Moran G, Noor N, Papamichael K, Peros G, Reenaers C, Sica G, Sigall-Boneh R, Vavricka SR, Yanai H, Myrelid P, Adamina M, Raine T. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. J Crohns Colitis. 2024;18:1531-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 95] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 2. | Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, Calabrese E, Baumgart DC, Bettenworth D, Borralho Nunes P, Burisch J, Castiglione F, Eliakim R, Ellul P, González-Lama Y, Gordon H, Halligan S, Katsanos K, Kopylov U, Kotze PG, Krustinš E, Laghi A, Limdi JK, Rieder F, Rimola J, Taylor SA, Tolan D, van Rheenen P, Verstockt B, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1169] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 3. | Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2677] [Cited by in RCA: 4108] [Article Influence: 513.5] [Reference Citation Analysis (110)] |
| 4. | Khalili H, Chan SSM, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 5. | Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 594] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 6. | Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 7. | Li A, Song NJ, Riesenberg BP, Li Z. The Emerging Roles of Endoplasmic Reticulum Stress in Balancing Immunity and Tolerance in Health and Diseases: Mechanisms and Opportunities. Front Immunol. 2019;10:3154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 8. | Coleman OI, Haller D. ER Stress and the UPR in Shaping Intestinal Tissue Homeostasis and Immunity. Front Immunol. 2019;10:2825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 9. | Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1177] [Cited by in RCA: 1141] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 10. | Bogaert S, De Vos M, Olievier K, Peeters H, Elewaut D, Lambrecht B, Pouliot P, Laukens D. Involvement of endoplasmic reticulum stress in inflammatory bowel disease: a different implication for colonic and ileal disease? PLoS One. 2011;6:e25589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Hooper KM, Barlow PG, Henderson P, Stevens C. Interactions Between Autophagy and the Unfolded Protein Response: Implications for Inflammatory Bowel Disease. Inflamm Bowel Dis. 2019;25:661-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78: A cell's response to stress. Life Sci. 2019;226:156-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 443] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 13. | Ma X, Dai Z, Sun K, Zhang Y, Chen J, Yang Y, Tso P, Wu G, Wu Z. Intestinal Epithelial Cell Endoplasmic Reticulum Stress and Inflammatory Bowel Disease Pathogenesis: An Update Review. Front Immunol. 2017;8:1271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, Luís A, McCarthy N, Montibeller L, More S, Papaioannou A, Püschel F, Sassano ML, Skoko J, Agostinis P, de Belleroche J, Eriksson LA, Fulda S, Gorman AM, Healy S, Kozlov A, Muñoz-Pinedo C, Rehm M, Chevet E, Samali A. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 672] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 15. | Beiranvand M. A review of the biological and pharmacological activities of mesalazine or 5-aminosalicylic acid (5-ASA): an anti-ulcer and anti-oxidant drug. Inflammopharmacology. 2021;29:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Li K, Zhu Y, Zhang P, Alini M, Grad S, Li Z. Anti-inflammatory and pro-anabolic effects of 5-aminosalicylic acid on human inflammatory osteoarthritis models. J Orthop Translat. 2023;38:106-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Zuo L, Ge S, Ge Y, Li J, Zhu B, Zhang Z, Jiang C, Li J, Wang S, Liu M, Li S, Wu R, Hu J. The Adipokine Metrnl Ameliorates Chronic Colitis in Il-10-/- Mice by Attenuating Mesenteric Adipose Tissue Lesions During Spontaneous Colitis. J Crohns Colitis. 2019;13:931-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Keubler LM, Buettner M, Häger C, Bleich A. A Multihit Model: Colitis Lessons from the Interleukin-10-deficient Mouse. Inflamm Bowel Dis. 2015;21:1967-1975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Hale LP, Greer PK. A novel murine model of inflammatory bowel disease and inflammation-associated colon cancer with ulcerative colitis-like features. PLoS One. 2012;7:e41797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 893] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 21. | Wen Z, Zhong Z, Darnell JE Jr. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1534] [Cited by in RCA: 1610] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 22. | Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1708] [Cited by in RCA: 1861] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 23. | Liu J, Zhou F, Chen Q, Kang A, Lu M, Liu W, Zang X, Wang G, Zhang J. Chronic inflammation up-regulates P-gp in peripheral mononuclear blood cells via the STAT3/Nf-κb pathway in 2,4,6-trinitrobenzene sulfonic acid-induced colitis mice. Sci Rep. 2015;5:13558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Zhang B, Xu Y, Liu S, Lv H, Hu Y, Wang Y, Li Z, Wang J, Ji X, Ma H, Wang X, Wang S. Dietary Supplementation of Foxtail Millet Ameliorates Colitis-Associated Colorectal Cancer in Mice via Activation of Gut Receptors and Suppression of the STAT3 Pathway. Nutrients. 2020;12:2367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Zhang M, Zhou L, Xu Y, Yang M, Xu Y, Komaniecki GP, Kosciuk T, Chen X, Lu X, Zou X, Linder ME, Lin H. A STAT3 palmitoylation cycle promotes T(H)17 differentiation and colitis. Nature. 2020;586:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 26. | Zhao L, Lv Y, Zhou X, Guo Z, Li H, Guo Y, Liu T, Tu L, Zhu L, Tao J, Shen G, He Y, Lei P. Secreted glucose regulated protein78 ameliorates DSS-induced mouse colitis. Front Immunol. 2023;14:986175. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |