Published online Feb 7, 2025. doi: 10.3748/wjg.v31.i5.102210
Revised: December 4, 2024
Accepted: December 16, 2024
Published online: February 7, 2025
Processing time: 68 Days and 0.3 Hours
Lymphovascular invasion (LVI) and perineural invasion (PNI) are associated with decreased survival in colorectal cancer (CRC), but its significance in N1c stage remains to be clearly defined.
To evaluate LVI and PNI as potential prognostic indicators in N1c CRC.
We retrospectively identified 107 consecutive patients who had CRC with N1c disease radically resected at our hospital. Tumors were reviewed for LVI and PNI by one pathologist blinded to the patients’ outcomes. Disease-free survival (DFS), overall survival (OS) and cancer-specific survival (CSS) were determined using the Kaplan-Meier method, with LVI and PNI prognosis differences determined by multivariate analysis using the Cox multiple hazards model. Results were compared using log-rank test. The receiver operating characteristic (ROC) curve was used to evaluate the prognostic predictive ability.
The median follow-up time was 63.17 (45.33-81.37) months for DFS, with 33.64% (36/107) of patients experiencing recurrence; 21.5% of tumors were found to be LVI positive and 44.9% PNI positive. The 5-year DFS rate was greater for patients with LVI-negative tumors compared with LVI-positive tumors (74.0% vs 35.6%), and PNI was similar (82.5% vs 45.1%). On multivariate analysis, LVI [hazard ratio (HR) = 3.368, 95% confidence interval (CI): 1.628-6.966, P = 0.001] and PNI (HR = 3.055, 95%CI: 1.478-6.313, P = 0.002) were independent prognostic factors for DFS. All patients could be divided into three groups of patients with different prognosis according to LVI and PNI. The 5-year ROC curve for LVI, PNI and their combination prediction of DFS was 0.646, 0.709 and 0.759, respectively. Similar results were seen for OS and CSS.
LVI and PNI could serve as independent prognostic factors of outcomes in N1c CRC patients. Patients with LVI or PNI should be given more attention during treatment.
Core Tip: This study evaluated lymphovascular invasion (LVI) and perineural invasion (PNI) as prognostic indicators in N1c colorectal cancer. Among 107 N1c patients, 21.5% were LVI-positive and 44.9% were PNI-positive. Five-year disease-free survival (DFS) rates were higher for LVI-negative (74.0%) and PNI-negative (82.5%) patients than LVI-positive (35.6%) and PNI-positive (45.1%) patients. On multivariate analysis, both LVI and PNI were independent prognostic factors. Receiver operating characteristic curve analysis showed good predictive ability for DFS, overall survival and cancer-specific survival. Patients with LVI or PNI should be closely monitored during treatment.
- Citation: Sun ZG, Chen SX, Sun BL, Zhang DK, Sun HL, Chen H, Hu YW, Zhang TY, Han ZH, Wu WX, Hou ZY, Yao L, Jie JZ. Important role of lymphovascular and perineural invasion in prognosis of colorectal cancer patients with N1c disease. World J Gastroenterol 2025; 31(5): 102210
- URL: https://www.wjgnet.com/1007-9327/full/v31/i5/102210.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i5.102210
Colorectal cancer (CRC), as one of the most prevalent malignancies of the digestive system worldwide, poses a significant threat to human life and health due to its high incidence and mortality rates[1]. CRC with N1c staging indicates the presence of tumor deposits (TDs) in the peri-colorectal fat or mesentery without direct evidence of lymph node metastasis (LNM), according to the eighth American Joint Committee on Cancer tumor node metastasis (TNM) staging system[2]. We began to stage TD (+) LNM (-) as N1c since 2017. Patients at stage N1c often face a heightened risk of recurrence and metastasis[3,4]. However, the prognostic factors affecting N1c staging patients are still unclear.
Risk grouping based on lymphovascular invasion (LVI) and perineural invasion (PNI) positivity is a strong predictor of disease-free survival (DFS) in patients with CRC[5]. LVI and PNI are also higher-risk factors for TNM stage II CRC. LVI and PNI have a detrimental effect on survival after diagnosis of stage II adenocarcinoma of the colon. Chemotherapy may be protective specifically when LVI and PNI are present[6]. Research about LVI and PNI in the prognosis of CRC patients with N1c disease is limited.
This study aimed to retrospectively analyze the clinicopathological information of patients with stage N1c CRC to explore the relationship between LVI, PNI and prognosis. We aimed to draw attention of researchers to the prognostic factors of N1c CRC, fostering collaborative efforts to advance the field of CRC diagnosis and treatment.
We included consecutive patients who were pathologically diagnosed with primary CRC and underwent curative surgical resection at China–Japan Friendship Hospital between July 2015 and July 2021. The inclusion criteria were: (1) Patients underwent radical resection and were diagnosed with malignant tumor by postoperative pathology reports; (2) Patients with complete clinicopathological data and follow-up information; and (3) TD information in postoperative pathology reports of patients could be obtained. The exclusion criteria were: (1) Patients had metastasis or died within 3 months after surgery; (2) Patients with distant metastasis at the time of first diagnosis; (3) Follow-up time < 2 years; and (4) Patients accepted neoadjuvant chemoradiotherapy or neoadjuvant chemotherapy before surgery.
Within this prospectively established cohort, electronic medical records including pathology reports were retrospectively reviewed. Baseline clinical data, such as age, sex, date of operation, tumor location, receipt of neoadjuvant chemotherapy, receipt of neoadjuvant chemoradiotherapy and information on distant metastasis. The clinical TNM staging of patients was evaluated by two medical imaging physicians via preoperative computed tomography (CT) or magnetic resonance imaging (MRI). In the pathology reports, the number of positive lymph nodes, number of total lymph nodes, number of negative lymph nodes, number of TDs, LVI, PNI, information on cell differentiation, tumor epigenetic type, tumor size, depth of invasion or pT stage, microsatellite instability status and lynch syndrome were reviewed. Patients with incom
Survival status regarding recurrence and/or death was collected by telephone call with patients/their family members, or from electronic medical records. DFS was estimated from the day of primary surgery to the date of diagnosis of recurrent disease or the last date of follow-up without recurrence or the date of death from other cause. Overall survival (OS) was estimated from the day of primary surgery to death from any cause or the last day of follow-up for those who were alive. Cancer-specific survival (CSS) was estimated from the day of primary surgery to death from tumor cause or the last day of follow-up for those who were alive.
All patients underwent radical surgery via total mesorectal excision for rectal cancer and complete mesocolic excision for colon cancer. Adjuvant chemotherapy with the CAPOX (capecitabine + oxaliplatin) or FOLFOX (fluorouracil + oxaliplatin + leucovorin) regimen was applied over six cycles, or adjuvant chemoradiotherapy was recommended if necessary. After surgery, the patients were followed up in the outpatient clinic every 3 months for the first 2 years, every 6 months for the next three years, and then annually until 10 years according to the National Comprehensive Cancer Network guidelines. At every visit, interim clinical history and laboratory blood test including carcinoembryonic antigen levels were checked. Abdominopelvic CT, chest CT and pelvic MRI in patients with rectal cancer were evaluated every 6 months.
Statistical differences between the groups were calculated using the χ2 test or Fisher’s exact test for categorical variables and Student's t test or Mann-Whitney U test for continuous variables. The cutoff value was calculated by the R package “survminer”. To compare the survival differences including DFS, OS and CSS between the target groups, Kaplan-Meier curves were plotted, and the log-rank test was performed. Univariable and multivariable regression analyses were per
A total of 107 patients with stage N1c CRC underwent curative surgery during the study period. The clinicopathological characteristics were compared between different groups based on LVI and PNI (Table 1). LVI was detected in 23 (21.5%) patients. PNI was detected in 48 (44.9%) patients and 15 (31.3%) had LVI. There were 52 (48.6%) male and 55 (51.4%) female patients. Most patients (97, 90.7%) had pT2 or pT3 stage, while 10 (9.3%) had pT4a or pT4b stage. Mucinous adenocarcinoma was found in 15 (14.0%) patients. Eighty-nine (83.2%) patients had > 12 lymph nodes obtained by postoperative pathology. Most number of TD was one (73, 68.2%). There were no significant differences in clinicopathological characteristics between patients with or without LVI and between those with or without PNI, including: Poorly differentiated tumor, pT stage, sex, mucinous adenocarcinoma, number of TDs, age and number of lymph nodes. Only between LVI and PNI, there was some certain correlation (χ2 = 4.909, P = 0.027) (Table 1).
| LVI (no) | LVI (yes) | χ2 | P value | PNI (no) | PNI (yes) | χ2 | P value | |
| PNI | 4.909 | 0.027 | ||||||
| No | 51 | 8 | ||||||
| Yes | 33 | 15 | ||||||
| Age (years) | 3.259 | 0.071 | 0.144 | 0.704 | ||||
| ≤ 50 | 7 | 5 | 6 | 6 | ||||
| > 50 | 77 | 18 | 53 | 42 | ||||
| Sex | 0.150 | 0.699 | 1.081 | 0.299 | ||||
| Female | 44 | 11 | 33 | 22 | ||||
| Male | 40 | 12 | 26 | 26 | ||||
| cT stage | 1.952 | 0.377 | 0.068 | 0.967 | ||||
| 2 | 10 | 5 | 8 | 7 | ||||
| 3 | 51 | 13 | 36 | 28 | ||||
| 4 | 1 | 1 | 1 | 1 | ||||
| cN stage | 0.209 | 0.901 | 9.349 | 0.009 | ||||
| 0 | 14 | 5 | 9 | 10 | ||||
| 1 | 33 | 9 | 19 | 23 | ||||
| 2 | 15 | 5 | 17 | 3 | ||||
| Poorly differentiated | 1.606 | 0.205 | 0.358 | 0.550 | ||||
| No | 77 | 19 | 52 | 44 | ||||
| Yes | 7 | 4 | 7 | 4 | ||||
| pT stage | 0.864 | 0.353 | 0.105 | 0.746 | ||||
| 2 and 3 | 75 | 22 | 53 | 44 | ||||
| 4 | 9 | 1 | 6 | 4 | ||||
| Mucinous adenocarcinoma | 0.276 | 0.599 | 0.937 | 0.333 | ||||
| No | 73 | 19 | 49 | 43 | ||||
| Yes | 11 | 4 | 10 | 5 | ||||
| TD (n) | 0.122 | 0.727 | 1.844 | 0.175 | ||||
| = 1 | 58 | 15 | 37 | 36 | ||||
| > 1 | 26 | 8 | 22 | 12 | ||||
| NLN (n) | 0.506 | 0.477 | 0.231 | 0.631 | ||||
| > 12 | 71 | 18 | 50 | 39 | ||||
| ≤ 12 | 13 | 5 | 9 | 9 |
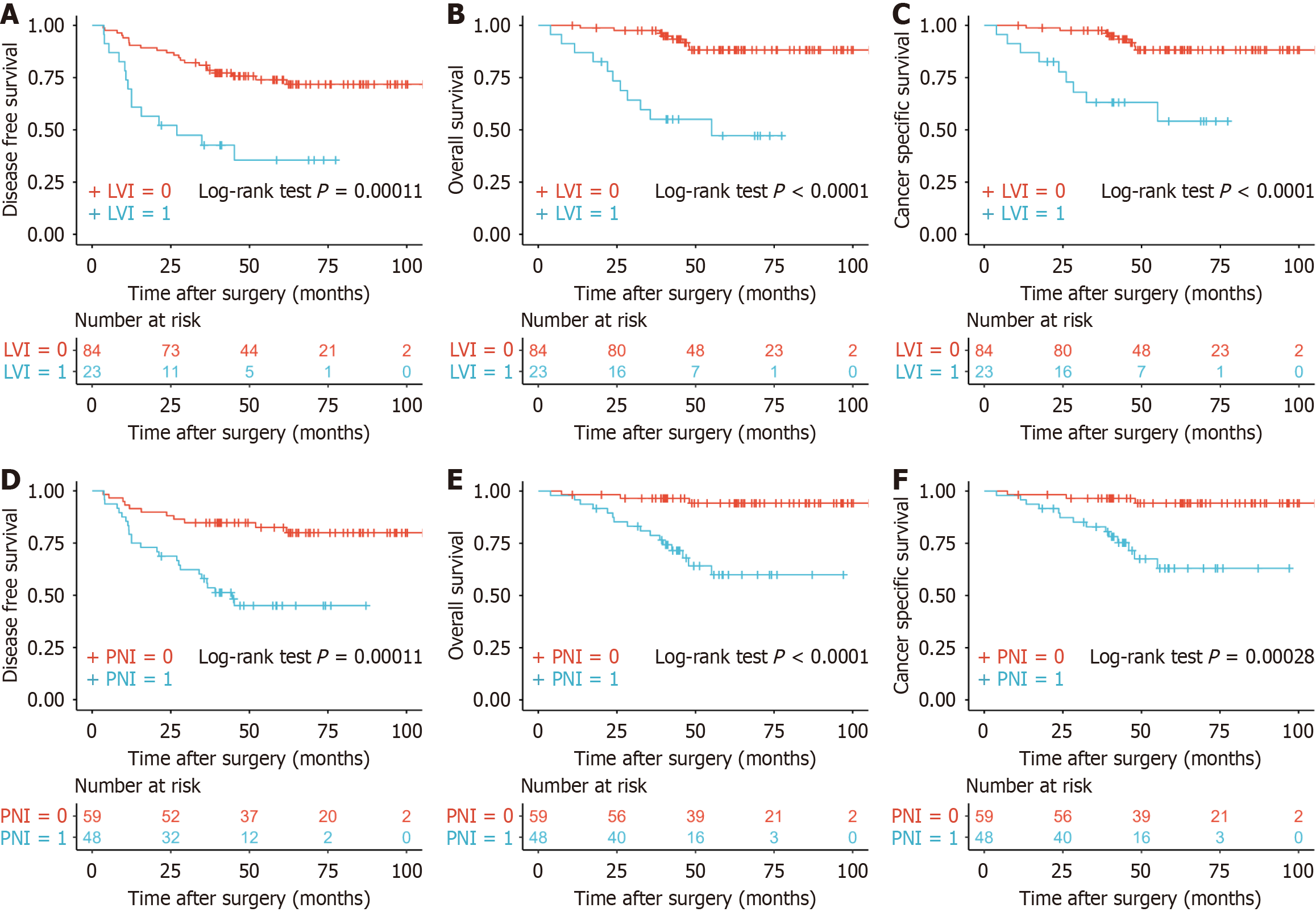
The median follow-up time for DFS, OS and CSS was 63.17 (45.33-81.37) months, 60.83 (44.0-77.40) months and 60.53 (42.83-77.40) months, respectively. Five-year DFS was 66.0% (57.3%-76.0%); 5-year OS was 79.5% (71.5%-88.4%); and 5-year CSS was 81.2% (73.4%-89.9%). Five-year DFS was 35.6% (19.5%-64.8%) for LVI-positive patients and 74.0% (64.9%-84.3%) for LVI-negative patients. Five-year DFS 45.1% (32.3%-62.8%) for PNI-positive patients and 82.5% (73.1%-93.0%) for PNI-negative patients. In the univariable regression analysis for DFS, LVI and PNI were significantly associated with DFS (Table 2). In the multivariable regression analysis for DFS, LVI and PNI were confirmed as independent risk factors (Table 3). OS and CSS showed similar results.
| DFS | OS | CSS | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| LVI | < 0.001 | < 0.001 | < 0.001 | |||
| No | 1 | 1 | 1 | |||
| Yes | 3.510 (1.785-6.902) | 7.373 (2.947-18.45) | 6.040 (2.314-15.77) | |||
| PNI | < 0.001 | < 0.001 | 0.002 | |||
| No | 1 | 1 | 1 | |||
| Yes | 3.753 (1.830-7.700) | 8.259 (2.393-28.50) | 7.320 (2.090-25.64) | |||
| Age (years) | 0.506 | 0.393 | 0.459 | |||
| ≤ 50 | 1 | 1 | 1 | |||
| > 50 | 1.494 (0.458-4.873) | 2.407 (0.321-18.03) | 2.144 (0.284-16.17) | |||
| Sex | 0.320 | 0.627 | 0.330 | |||
| Female | 1 | 1 | 1 | |||
| Male | 1.397 (0.723-2.696) | 1.251 (0.508-3.081) | 1.617 (0.615-4.253) | |||
| cT stage | 0.414 | 0.392 | 0.392 | |||
| 2 | 1 | 1 | 1 | |||
| 3 | 1.360 (0.469-3.949) | 0.572 | 1.231 (0.270-5.627) | 0.788 | 1.231 (0.270-5.627) | 0.788 |
| 4 | 1.539 (0.511-4.634) | 4.634 | 2.224 (0.663-7.466) | 0.196 | 2.224 (0.663-7.466) | 0.196 |
| cN stage | 0.869 | 0.733 | 0.733 | |||
| 0 | 1 | 1 | 1 | |||
| 1 | 2.446 (0.826-7.238) | 0.106 | 1.998 (0.423-9.438) | 0.382 | 1.998 (0.423-9.438) | 0.382 |
| 2 | 1.067 (0.553-2.060) | 0.847 | 1.180 (0.482-2.887) | 0.717 | 1.180 (0.482-2.887) | 0.717 |
| Poorly differentiated | 0.280 | 0.204 | 0.550 | |||
| No | 1 | 1 | 1 | |||
| Yes | 1.687 (0.653-4.357) | 2.228 (0.647-7.678) | 1.570 (0.358-6.896) | |||
| pT stage | 0.035 | 0.599 | 0.490 | |||
| 2 and 3 | 1 | 1 | 1 | |||
| 4 | 2.580 (1.070-6.220) | 1.482 (0.342-6.421) | 1.682 (0.384-7.364) | |||
| Mucinous adenocarcinoma | 0.209 | 0.224 | 0.267 | |||
| No | 1 | 1 | 1 | |||
| Yes | 0.468 (0.143-1.529) | 0.286 (0.038-2.149) | 0.318 (0.042-2.406) | |||
| TD (n) | 0.168 | 0.365 | 0.470 | |||
| = 1 | 1 | 1 | 1 | |||
| > 1 | 1.603 (0.820-3.134) | 1.540 (0.605-3.919) | 1.444 (0.533-3.914) | |||
| NLN (n) | 0.063 | 0.194 | 0.116 | |||
| > 12 | 1 | 1 | 1 | |||
| ≤ 12 | 2.045 (0.961-4.351) | 1.969 (0.709-5.469) | 2.308 (0.813-6.554) | |||
| DFS | OS | CSS | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| LVI | 0.001 | < 0.001 | 0.003 | |||
| No | 1 | 1 | 1 | |||
| Yes | 3.368 (1.628-6.966) | 5.972 (2.184-16.33) | 4.933 (1.736-14.02) | |||
| PNI | 0.002 | 0.007 | 0.011 | |||
| No | 1 | 1 | 1 | |||
| Yes | 3.055 (1.478-6.313) | 5.643 (1.615-19.72) | 5.170 (1.459-18.32) | |||
| pT stage | 0.009 | 0.239 | 0.252 | |||
| 2 and 3 | 1 | 1 | 1 | |||
| 4 | 3.523 (1.378-9.009) | 2.621 (0.526-13.05) | 2.561 (0.513-12.80) | |||
| NLN (n) | 0.171 | 0.333 | 0.233 | |||
| ≥ 12 | 1 | 1 | 1 | |||
| < 12 | 1.698 (0.796-3.623) | 1.668 (0.592-4.698) | 1.904 (0.661-5.482) | |||
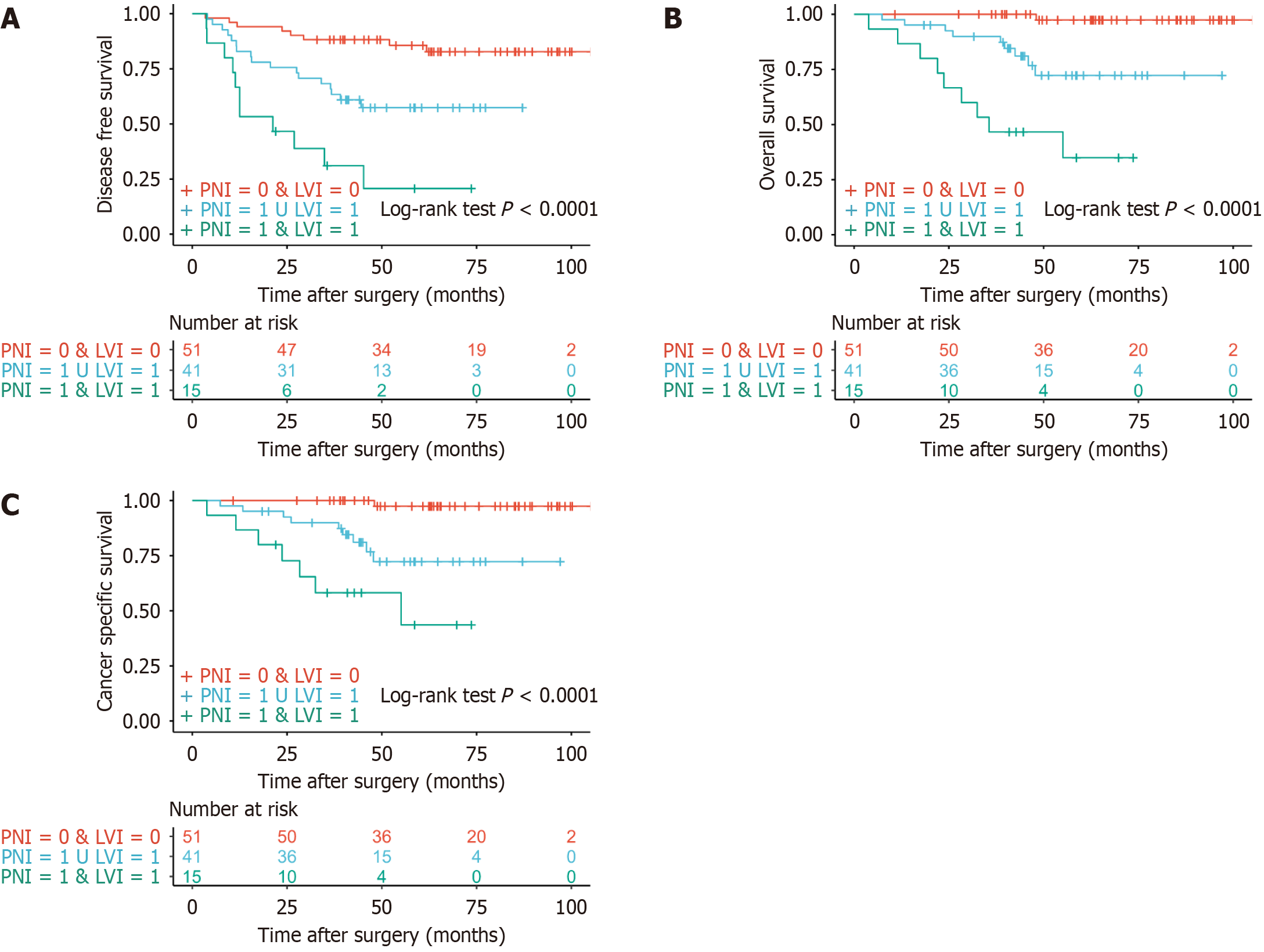
There were significant differences in prognosis between patients with and without LVI for DFS, OS and CSS (all P < 0.001) (Figure 1A-C). PNI had similar results for DFS, OS and CSS (all P < 0.001) (Figure 1D-F). All patients were divided into three groups according to LVI and PNI, patients without LVI and PNI, patients with LVI or PNI, and patients with LVI and PNI. The prognosis for DFS was separated into three possible outcomes from the Kaplan-Meier survival curves
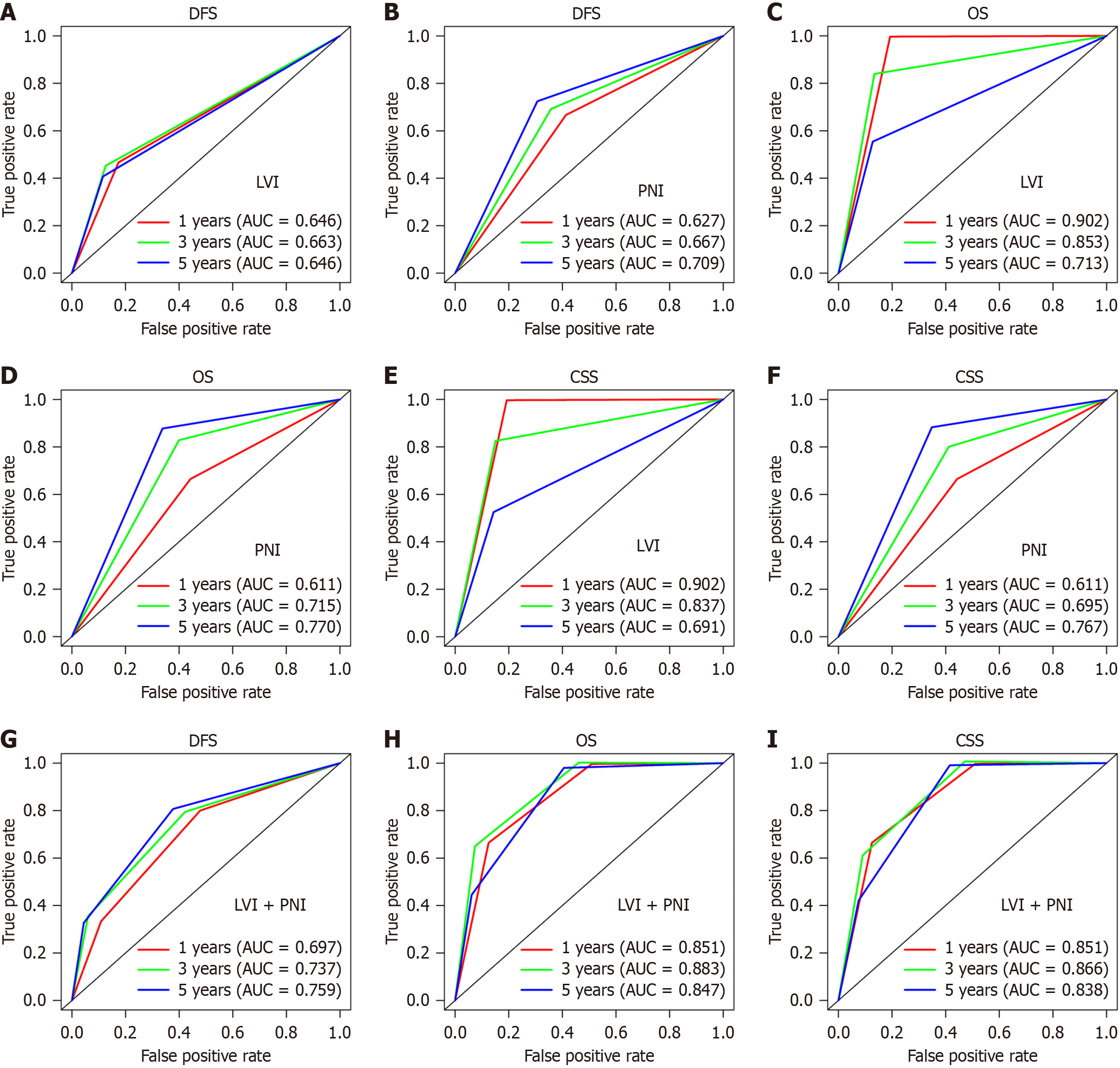
The 1-year area under the receiver operating characteristic (ROC) curve (AUC) for patients with or without LVI was 0.646, 3-year AUC was 0.663 and 5-year AUC was 0.646 for DFS (Figure 3A). The 1-year AUC for patients with or without PNI was 0.627, 3-year AUC was 0.667, and 5-year AUC was 0.709 for DFS (Figure 3B). The results for OS and CSS were 0.902, 0.853, 0.713, 0.611, 0.715 and 0.770 (Figure 3C and D) and 0.902, 0.837, 0.691, 0.611, 0.695 and 0.767 (Figure 3E and F), respectively. The one-year AUC for these three groups based on LVI and PNI was 0.697, 3-year AUC was 0.737 and 5-year area was 0.759 for DFS (Figure 3G). The results for OS and CSS were 0.851, 0.883 and 0.847, and 0.851, 0.866 and 0.838, respectively (Figure 3H and I).
This study had comprehensively analyzed the impact of LVI and PNI on the prognosis of CRC patients with N1c disease status. Both LVI and PNI have emerged as significant factors influencing the survival outcomes and disease progression in these patients. Our findings underscore the importance of considering these pathological features in clinical decision-making and risk stratification.
CRC with N1c staging indicates the presence of TDs without direct evidence of LNM[2]. TDs were redefined as discrete tumor nodules within lymphatic drainage areas of the primary carcinoma, without identifiable lymph nodes or vascular or neural structures. TD-positive rates ranging from 9.5% to 26.9% have been reported[7-12]. The presence of TDs has been confirmed to have adverse effects on prognosis[13], with or without LNM[8,14,15] and with or without receiving neoadjuvant chemoradiotherapy[16].
LVI refers to the formation of tumor cell clusters within blood vessels or lymphatic channels, obstructing the lumen and potentially leading to ischemia, hypoxia, or even necrosis in distal tissues or organs. Tumor LVI represents a critical step during malignant progression of epithelial cancers[17]. This pathological phenomenon not only reflects the aggressive and metastatic potential of tumor cells but also predicts poor prognosis[6]. Lymphatic spread of cancer cells via the lymphatic channels or venules may be a critical early step for LNM[18]. Experimental research via single-cell RNA sequencing and spatial transcriptomics in early-stage cancer, elucidating the critical role about promoting lymphangiogenesis, LVI, and LNM, thus shaping the metastatic landscape and guiding treatment strategies for early-stage cancer[19]. Disruption of the lymphatic endothelial barrier increases lymphatic vessel permeability and makes it easier for cancer cells to invade[20]. Lymphatic endothelial cells play important role via ligand-receptor interactions. Tumor cells interact with lymphatic endothelial cells through specific ligand-receptor interactions, such as ITGA11-SELE, enhancing their transport within the lymphatic vessels. These interactions may activate signaling pathways such as SRC-p-vascular endothelial growth factor receptor 3-mitogen-activated protein kinase that promote lymphangiogenesis, facilitating the metastasis of tumor cells[19].
The existence of LVI, as direct evidence of tumor invasion into the vascular system, undoubtedly intensifies this risk. Consequently, a thorough investigation into the prognostic role of LVI in patients with stage N1c CRC is imperative. Similar to this study, risk grouping based on LVI, PNI and tumor budding positivity is a strong predictor of DFS in patients with stage I-III CRC[5].
PNI refers to the infiltration of cancer cells into the perineural spaces, surrounding or invading the nerve sheaths. This phenomenon has been observed in various malignancies, including CRC, and is associated with increased local recurrence, distant metastasis, and reduced survival. The contribution of nerves to the pathogenesis of malignancies has emerged as an important component of the tumor microenvironment[21]. The prognostic value of PNI is similar to that of well-established prognostic factors such as depth of invasion, differentiation grade, LNM, and lymphatic and extramural vascular invasion. Therefore, PNI should be one of the factors in the standardized reporting of CRC and might be considered a high-risk feature[22]. PNI is a common feature in various malignancies and is associated with tumor invasion, metastasis, cancer-related pain, and unfavorable clinical outcomes[23]. PNI can disrupt the normal structure and function of nerve fibers, potentially leading to changes in nerve signal transduction[24]. It is possible that PNI facilitates the spread of cancer cells through nerve fibers, but this remains a controversial area of research. PNI is the process through which cancer cells invade the perineural spaces of surrounding nerves and is not simply the movement of cancer cells along a path of low resistance. PNI is a directed process that involves many signaling molecules from various pathways; these signaling molecules are produced by both the cancer cells and the nerves. This constitutes a means for the cancer cells to spread to distant locations[25]. PNI may be mediated by nerve secretion of glial cell line-derived neurotrophic factor attracting cancer cell migration through activation of cell surface ret proto-oncogene receptors[26].
The results of our study confirm the strong association between LVI, PNI and adverse prognosis in CRC patients. LVI, or the presence of cancer cells within the lymphatic or vascular spaces, signifies aggressive tumor biology and a higher likelihood of metastatic spread. This is in line with previous studies that have reported LVI and PNI as independent prognostic factors in various stages of CRC[27,28]. Our analysis revealed that LVI- or PNI-positive patients had significantly lower survival rates compared to LVI- or PNI-negative patients in N1c stage, indicating the detrimental effect of LVI and PNI on patient outcomes. This finding underscores the need for more aggressive therapeutic approaches and closer follow-up in LVI- or PNI-positive patients.
The results of our study have important implications for clinical practice. Pathologists should carefully evaluate surgical specimens for the presence of LVI and PNI, as this information can inform patient management and prognostication. Clinicians should consider LVI and PNI status in their treatment recommendations and follow-up strategies, ensuring that patients with these risk factors receive appropriate and timely interventions. We hope to elucidate the specific mechanisms underlying the prognostic significance of LVI, PNI in stage N1c CRC, thereby providing a scientific basis for more personalized treatment plans and enhancing patient survival and quality of life.
LVI and PNI could serve as independent prognostic factors of outcomes in N1c CRC patients.
We would like to express our gratitude to Zhang HZ, Feng L and Dong XS, for their invaluable guidance, patience and continuous support throughout this research project. Their expert advice, rigorous scrutiny and encouragement have been instrumental in shaping this work and fostering my academic growth.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64722] [Article Influence: 16180.5] [Reference Citation Analysis (177)] |
| 2. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4410] [Article Influence: 551.3] [Reference Citation Analysis (4)] |
| 3. | Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, Ueno H, Quirke P. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol. 2017;35:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 4. | Bouquot M, Creavin B, Goasguen N, Chafai N, Tiret E, André T, Flejou JF, Parc Y, Lefevre JH, Svrcek M. Prognostic value and characteristics of N1c colorectal cancer. Colorectal Dis. 2018;20:O248-O255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 5. | Huh JW, Lee WY, Shin JK, Park YA, Cho YB, Kim HC, Yun SH. A novel histologic grading system based on lymphovascular invasion, perineural invasion, and tumor budding in colorectal cancer. J Cancer Res Clin Oncol. 2019;145:471-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA. Lymphovascular Invasion and Perineural Invasion Negatively Impact Overall Survival for Stage II Adenocarcinoma of the Colon. Dis Colon Rectum. 2019;62:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 7. | Pyo DH, Kim SH, Ha SY, Yun SH, Cho YB, Huh JW, Park YA, Shin JK, Lee WY, Kim HC. Revised Nodal Staging Integrating Tumor Deposit Counts With Positive Lymph Nodes in Patients With Stage III Colon Cancer. Ann Surg. 2023;277:e825-e831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 8. | Agger E, Jörgren F, Jöud A, Lydrup ML, Buchwald P. Negative Prognostic Impact of Tumor Deposits in Rectal Cancer: A National Study Cohort. Ann Surg. 2023;278:e526-e533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 9. | Cohen R, Shi Q, Meyers J, Jin Z, Svrcek M, Fuchs C, Couture F, Kuebler P, Ciombor KK, Bendell J, De Jesus-Acosta A, Kumar P, Lewis D, Tan B, Bertagnolli MM, Philip P, Blanke C, O'Reilly EM, Shields A, Meyerhardt JA. Combining tumor deposits with the number of lymph node metastases to improve the prognostic accuracy in stage III colon cancer: a post hoc analysis of the CALGB/SWOG 80702 phase III study (Alliance)(☆). Ann Oncol. 2021;32:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 10. | Delattre JF, Cohen R, Henriques J, Falcoz A, Emile JF, Fratte S, Chibaudel B, Dauba J, Dupuis O, Bécouarn Y, Bibeau F, Taieb J, Louvet C, Vernerey D, André T, Svrcek M. Prognostic Value of Tumor Deposits for Disease-Free Survival in Patients With Stage III Colon Cancer: A Post Hoc Analysis of the IDEA France Phase III Trial (PRODIGE-GERCOR). J Clin Oncol. 2020;38:1702-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 11. | Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E. Prognostic Significance of Tumor Deposits in Stage III Colon Cancer. Ann Surg Oncol. 2018;25:3179-3184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 12. | Zheng P, Chen Q, Li J, Jin C, Kang L, Chen D. Prognostic Significance of Tumor Deposits in Patients With Stage III Colon Cancer: A Nomogram Study. J Surg Res. 2020;245:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 13. | Jörgren F, Agger E, Lydrup ML, Buchwald P. Tumour deposits in colon cancer predict recurrence and reduced survival in a nationwide population-based study. BJS Open. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 14. | Moon JY, Lee MR, Ha GW. Prognostic value of tumor deposits for long-term oncologic outcomes in patients with stage III colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2022;37:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 15. | Lundström S, Agger E, Lydrup ML, Jörgren F, Buchwald P. Adverse impact of tumor deposits in lymph node negative rectal cancer - a national cohort study. Int J Colorectal Dis. 2023;38:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (2)] |
| 16. | Wang Y, Zhang J, Zhou M, Yang L, Wan J, Shen L, Liang L, Yao Y, Zhang H, Zhang Z. Poor prognostic and staging value of tumor deposit in locally advanced rectal cancer with neoadjuvant chemoradiotherapy. Cancer Med. 2019;8:1508-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 17. | Wolf B, Jain RK. Unraveling a hidden player in lymphovascular invasion in bladder cancer. Cancer Cell. 2024;42:509-512. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 586] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 19. | Zheng H, An M, Luo Y, Diao X, Zhong W, Pang M, Lin Y, Chen J, Li Y, Kong Y, Zhao Y, Yin Y, Ai L, Huang J, Chen C, Lin T. PDGFRα+ITGA11+ fibroblasts foster early-stage cancer lymphovascular invasion and lymphatic metastasis via ITGA11-SELE interplay. Cancer Cell. 2024;42:682-700.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 20. | Tacconi C, Correale C, Gandelli A, Spinelli A, Dejana E, D’Alessio S, Danese S. Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology. 2015;148:1438-51.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Zahalka AH, Frenette PS. Nerves in cancer. Nat Rev Cancer. 2020;20:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 22. | Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review. Am J Surg Pathol. 2016;40:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Wang H, Huo R, He K, Cheng L, Zhang S, Yu M, Zhao W, Li H, Xue J. Perineural invasion in colorectal cancer: mechanisms of action and clinical relevance. Cell Oncol (Dordr). 2024;47:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 24. | Saidak Z, Lailler C, Clatot F, Galmiche A. Perineural invasion in head and neck squamous cell carcinoma: background, mechanisms, and prognostic implications. Curr Opin Otolaryngol Head Neck Surg. 2020;28:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 26. | He S, Chen CH, Chernichenko N, He S, Bakst RL, Barajas F, Deborde S, Allen PJ, Vakiani E, Yu Z, Wong RJ. GFRα1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling. Proc Natl Acad Sci U S A. 2014;111:E2008-E2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Al-Sukhni E, Attwood K, Gabriel EM, LeVea CM, Kanehira K, Nurkin SJ. Lymphovascular and perineural invasion are associated with poor prognostic features and outcomes in colorectal cancer: A retrospective cohort study. Int J Surg. 2017;37:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Chen PC, Yeh YM, Lin BW, Chan RH, Su PF, Liu YC, Lee CT, Chen SH, Lin PC. A Prediction Model for Tumor Recurrence in Stage II-III Colorectal Cancer Patients: From a Machine Learning Model to Genomic Profiling. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |