Published online Jan 14, 2025. doi: 10.3748/wjg.v31.i2.98031
Revised: October 25, 2024
Accepted: November 18, 2024
Published online: January 14, 2025
Processing time: 185 Days and 16.2 Hours
Focal nodular hyperplasia (FNH)-like lesions are hyperplastic formations in patients with micronodular cirrhosis and a history of alcohol abuse. Although pathologically similar to hepatocellular carcinoma (HCC) lesions, they are benign. As such, it is important to develop methods to distinguish between FNH-like lesions and HCC.
To evaluate diagnostically differential radiological findings between FNH-like lesions and HCC.
We studied pathologically confirmed FNH-like lesions in 13 patients with alco
The evaluated lesion features included arterial enhancement pattern, washout appearance (low density compared with that of surrounding liver parenchyma), signal intensity on T1-weighted image (T1WI) and T2-weighted image (T2WI), central scar presence, chemical shift on in- and out-of-phase images, and uptake pattern on gadoxetic acid-enhanced MRI hepatobiliary phase and SPIO-enhanced MRI. Eleven patients had multiple small lesions (< 1.5 cm). Radiological features of FNH-like lesions included hypervascularity despite small lesions, lack of “corona-like” enhancement in the late phase on CT during hepatic angiography (CTHA), high-intensity on T1WI, slightly high- or iso-intensity on T2WI, no signal decrease in out-of-phase images, and complete SPIO uptake or incomplete/partial uptake of gadoxetic acid. Pathologically, similar to HCC, FNH-like lesions showed many unpaired arteries and sinusoidal capillarization.
Overall, the present study showed that FNH-like lesions have unique radiological findings useful for differential diagnosis. Specifically, SPIO- and/or gadoxetic acid-enhanced MRI and CTHA features might facilitate differential diagnosis of FNH-like lesions and HCC.
Core Tip: Two enhancement patterns were observed for the hepatobiliary phase on gadoxetic acid-enhanced magnetic resonance imaging (MRI): Heterogeneous hyperintense (43%) and ring-like enhancement (57%), and all lesions exhibited a marked homogeneous uptake pattern on superparamagnetic iron oxide-enhanced MRI in patients with focal nodular hyperplasia-like lesions. This finding is of clinical relevance because it is useful for the differential diagnosis of hypervascular liver nodules in patients with chronic liver disease.
- Citation: Urase A, Tsurusaki M, Kozuki R, Kono A, Sofue K, Ishii K. Imaging characteristics of hypervascular focal nodular hyperplasia-like lesions in patients with chronic alcoholic liver disease. World J Gastroenterol 2025; 31(2): 98031
- URL: https://www.wjgnet.com/1007-9327/full/v31/i2/98031.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i2.98031
Focal nodular hyperplasia (FNH)-like lesions were initially reported by Gluud et al[1] as hyperplastic formations in patients with micronodular cirrhosis and a history of alcohol abuse. These lesions, now widely identified in patients with alcoholic liver cirrhosis, are histopathologically similar to FNH in non-cirrhotic livers[2-7]. However, FNH-like lesions further exhibit unique vascular characteristics, including hypervascularity and sinusoidal capillarization[7], which are also common in hepatocellular carcinoma (HCC). Conversely, unlike HCC, FNH-like lesions are generally considered benign and non-progressive, particularly following alcohol abstinence. Despite these findings, FNH-like lesions are not yet classified in major diagnostic guidelines, such as those of the International Working Party[8] or the World Health Organization (WHO)[9], presenting challenges in diagnosis and clinical management. Clinically, the differentiation between FNH-like lesions and HCC is essential as the treatment approach differs significantly. While HCC commonly requires aggressive treatment options, including surgery, ablation, or systemic therapies, FNH-like lesions may not require any interventions, making an accurate diagnosis crucial for appropriate patient care. The current literature on FNH-like lesions primarily addresses their pathology, and there are relatively few studies on their imaging characteristics, making non-invasive diagnosis challenging.
This study aimed to address these gaps by examining the radiological features of FNH-like lesions in alcoholic liver cirrhosis using dynamic computed tomography (CT), superparamagnetic iron oxide (SPIO), and gadoxetic acid-enhanced magnetic resonance imaging (MRI). By identifying specific imaging criteria, we aimed to support non-invasive differentiation of FNH-like lesions from early-stage HCC, potentially reducing the need for invasive biopsy and providing clearer diagnostic pathways for clinicians.
This retrospective study was approved by the institutional ethics committee of Kindai University, Faculty of Medicine (ethical identification No. 27-067). All patients provided informed consent for the use of their CT and MRI, as well as their biopsy specimens. The study protocol conformed to the ethics guidelines of the 2002 Declaration of Helsinki.
This study enrolled 13 patients with pathologically confirmed FNH-like lesions who underwent dynamic CT, unenhanced MRI, and SPIO- and/or gadoxetic acid-enhanced MRI between 2000 and 2020. In all the patients, liver cirrhosis was determined through clinical examination and blood chemistry tests (for aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, bilirubin, albumin, and globulin). None of the patients included in this study had comorbidities such as diabetes, hypertension, or metabolic syndrome, as verified by assessment of their medical histories and clinical examinations. Further, each patient had at least one FNH-like nodule pathologically diagnosed through biopsy, with the remaining lesions being diagnosed based on clinical findings indicating no decrease in size after abstinence from alcohol during the 1-year follow-up.
Plain and three-phase contrast-enhanced CT (CE-CT) scans were obtained in a craniocaudal direction using Sensation 64 CT (Siemens, Erlangen, Germany) or Aquilion multi 64 (Canon Medical System, Otawara, Japan) scanners. Routine scanning was conducted at a 5-mm section thickness and 5-mm scan increment. Subsequently, the scans were recon
All MRIs were performed on a 1.5-T system using the Magnetom Avanto (Siemens Healthcare, Erlangen, Germany) or Signa HDxt (GE Healthcare, Waukesha, WI, United States) scanner. Baseline MRI were acquired using a respiratory-triggered T2-weighted turbo spin-echo (TSE) sequence, breath-hold T2*-weighted gradient-echo (GRE) imaging with steady-state precession (FISP) sequence, and breath-hold T1-weighted GRE sequence. All images were obtained in the transaxial plane, using a phased-array multi-coil. For all sequences, a 7-mm slice thickness was used, with a 10% intersection gap and a field of view of 35-40 cm, depending on the liver size. SPIO-enhanced MRI comprised the respiratory-triggered T2-TSE sequence, breath-hold T2*-weighted FISP sequence, and breath-hold T1-weighted GRE sequence, with the same parameters as those used in baseline MRI. Fercarbotran (Resovist®; Bayer Yakuhin, Osaka, Japan) at a dose of 8.0 μmol iron per kg (body weight) was manually injected as a rapid bolus through a filter with 5-μm pore size, which was immediately followed by a 10 mL saline solution flush. Imaging was then performed after 7 minutes. Gadoxetic acid-enhanced MRI comprised dynamic images constructed using fat-suppressed T1-weighted GRE images obtained before (pre-contrast) as well as 14-30 seconds (arterial phase), 70 seconds, and 3 minutes after intravenous administration of 0.025 mmol of gadoxetic acid (Primovist®, Bayer Schering Pharma, Berlin, Germany) per kg (body weight) at a rate of 2.0 mL/s, followed by a 20 mL saline flush. Hepatobiliary phase (HBP) images were obtained 20 minuntes after gadoxetic acid injection.
All CT during arterial portography and double-phase CT during hepatic angiography (CTHA) examinations were performed using a unified angiography and CT system (Multistar Plus/Somatom Plus 4 Volume Zoom; Siemens, Erlangen, Germany). These images were subsequently obtained in a craniocaudal direction with 5-mm section thickness and 5-mm reconstruction intervals. For double-phase CTHA, first-phase data acquisition was started 5 seconds after second-phase data acquisition, which was performed 21-26 seconds after initiation of a transcatheter hepatic arterial injection of a fixed dose of 20 mL iohexol (Omnipaque 300, 300 mgI/mL; Daiichi-Sankyo Parma, Tokyo, Japan) diluted with 33% sterile water (iodine, 100 mg iodine/mL) at a rate of 2 mL/s.
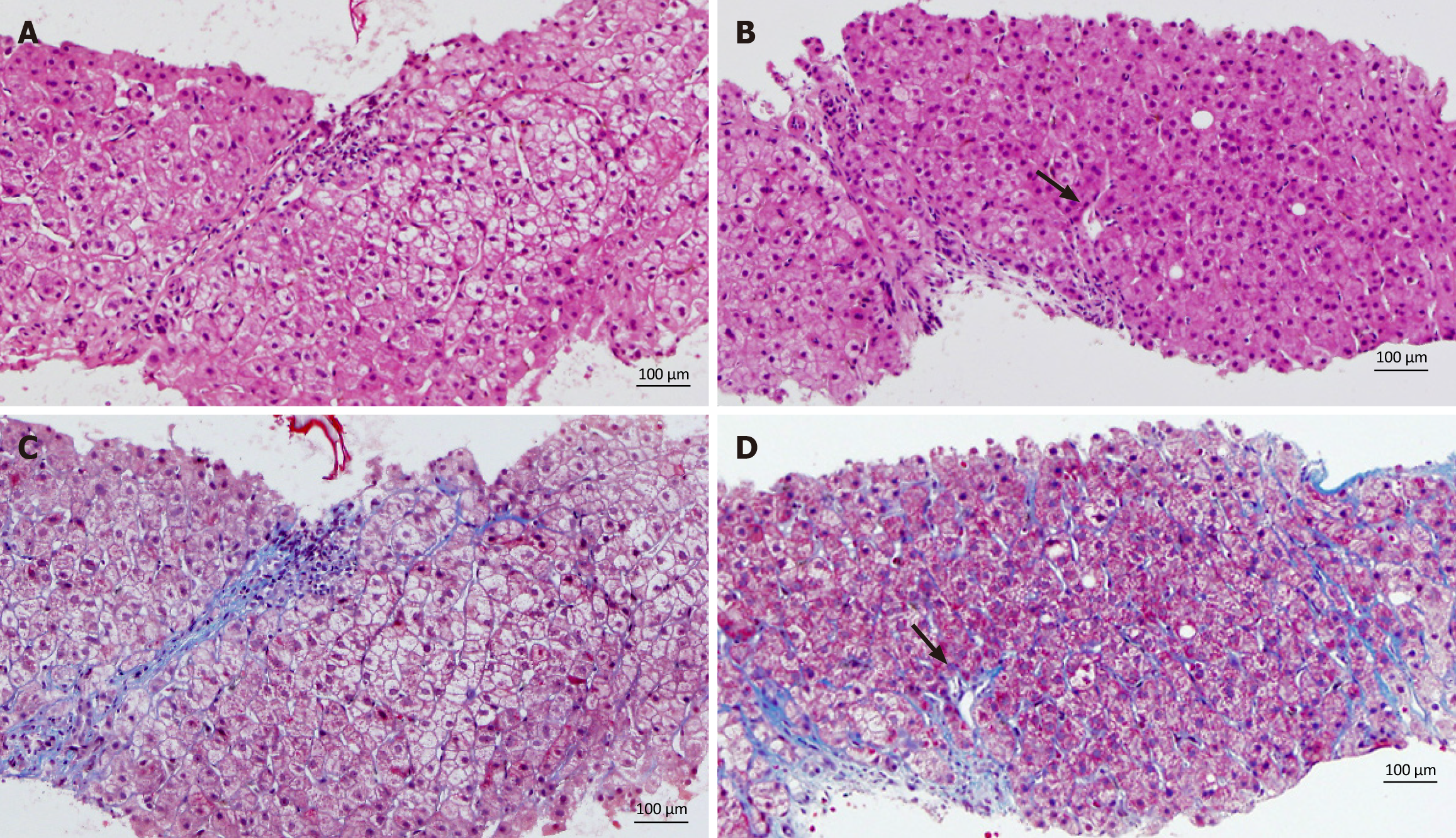
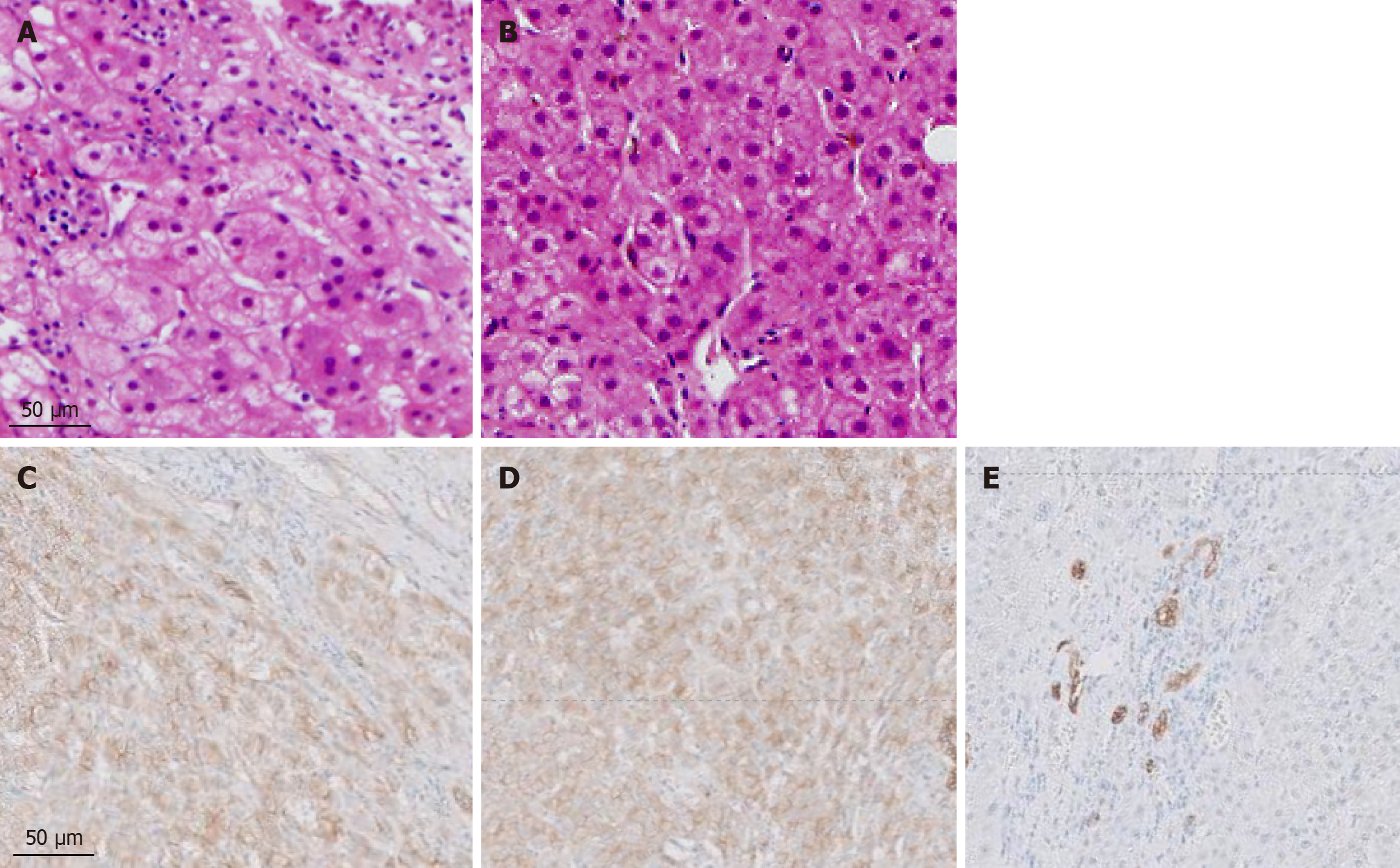
All patients underwent ultrasound-guided core needle biopsy, following which the liver samples were fixed in 10% neutral-buffered formalin, routinely processed for paraffin embedding, sectioned (4 µm), and stained with hematoxylin and eosin or Masson trichrome (Muto Pure Chemicals, Tokyo, Japan) according to standard procedures. Immunohistochemical analysis for OATP8 and CD68 antigens were performed on formalin-fixed, paraffin-embedded specimens extracted from seven patients. The stained sections were evaluated under a microscope. The expressions of OATP8 and CD68 antigen were typically assessed by determining the intensity and localization of staining within hepatocytes. The intensity was graded semi-quantitatively (as either none, weak, moderate, or strong), and the localization was confirmed as predominantly cytoplasmic and/or membrane-associated, depending on the known expression pattern of OATP8. The percentage of positively stained cells was also recorded.
Two radiologists with > 10 years of experience evaluated the following lesion characteristics: Number and size (largest axial diameter) of each lesion; dynamic enhancement pattern on dynamic CT and CTHA (1 and 2) for qualitative imaging analysis; and other MRI features (3-8) as follows: (1) Arterial enhancement pattern of the dynamic CT and/or first phase of the CTHA; (2) Washout appearance (defined as low density compared with the density of the surrounding liver parenchyma) on the portal venous phase (PVP) of the dynamic CT and/or second phase of the CTHA; (3) Signal intensity on T1-weighted image (T1WI); (4) Signal intensity on T2-weighted image (T2WI); (5) Central scar (defined as a central T2 hyperintensity and/or T1 hypointensity representing fibrotic tissue); (6) Chemical shift (defined as a drop in signal intensity on out-of-phase images compared with that on in-phase images); (7) Uptake pattern on the HBP of the gadoxetic acid-enhanced MRI; and (8) Uptake pattern on the SPIO-enhanced MRI. Arterial enhancement was classified as homogeneous (peripherally or entirely) or heterogeneous. Signal intensity on T1WIs was classified as hypointensity, iso- or hyperintensity, or heterogeneous signal intensity. The signal intensity on T2WIs was classified as iso-intensity or hyperintensity. Uptake on the HBP of gadoxetic acid- and SPIO-enhanced MRI was classified as homogeneous (peripherally or entirely) or heterogeneous. In case of interobserver discordance, the radiologists re-evaluated the images and reached a consensus. Both radiologists were informed about the location of each lesion; however, they were blinded to the clinical information and final diagnosis.
This study included 13 patients [10 men and 3 women; mean age: 54.5 ± 12.5 (33-72) years] with chronic liver failure due to alcoholic liver disease. Table 1 presents a summary of the clinical findings. All patients with FNH-like lesions were pathologically diagnosed using fine-needle biopsy. The levels of alpha-fetoprotein (AFP; ng/mL) and prothrombin induced by vitamin K deficiency or antagonist-II (mAU/mL) were normal in all patients with FNH-like lesions, except for one patient with HCC who showed elevated AFP (ng/mL) levels. Among the 10 patients with FNH-like lesions, 2 (20%) showed new lesions following abstinence from alcohol. The size of the FNH-like lesions remained stable or decreased in eight (80%) patients.
| Patient No. | Age (years) | Sex | Background of liver failure | Child Pugh grade | AFP | PIVKA-II | Diagnosis | Lesion growth at imaging follow up |
| 1 | 41 | M | Alcoholic | A | 6 | 23 | FNB | N/A |
| 2 | 33 | F | Alcoholic | A | 7 | 23 | FNB | No growth |
| 3 | 34 | F | Alcoholic | B | 4 | 10 | FNB | Increased number |
| 4 | 60 | M | Alcoholic | B | 9 | 20 | FNB | No growth |
| 5 | 58 | M | Alcoholic | A | 7 | 40 | FNB | No growth |
| 6 | 57 | M | Alcoholic | B | 6 | 19 | FNB | N/A |
| 7 | 53 | M | Alcoholic | B | 231 | 33 | FNB | No growth |
| 8 | 65 | M | Alcoholic | A | 10 | 18 | FNB | Increased number |
| 9 | 63 | M | Alcoholic | A | 6 | 12 | FNB | No growth |
| 10 | 48 | F | Alcoholic | B | 11 | 18 | FNB | No growth |
| 11 | 55 | M | Alcoholic | B | 5 | 16 | FNB | N/A |
| 12 | 70 | M | Alcoholic | A | 5 | 22 | FNB | No growth |
| 13 | 72 | F | Alcoholic | A | 4 | 15 | FNB | No growth |
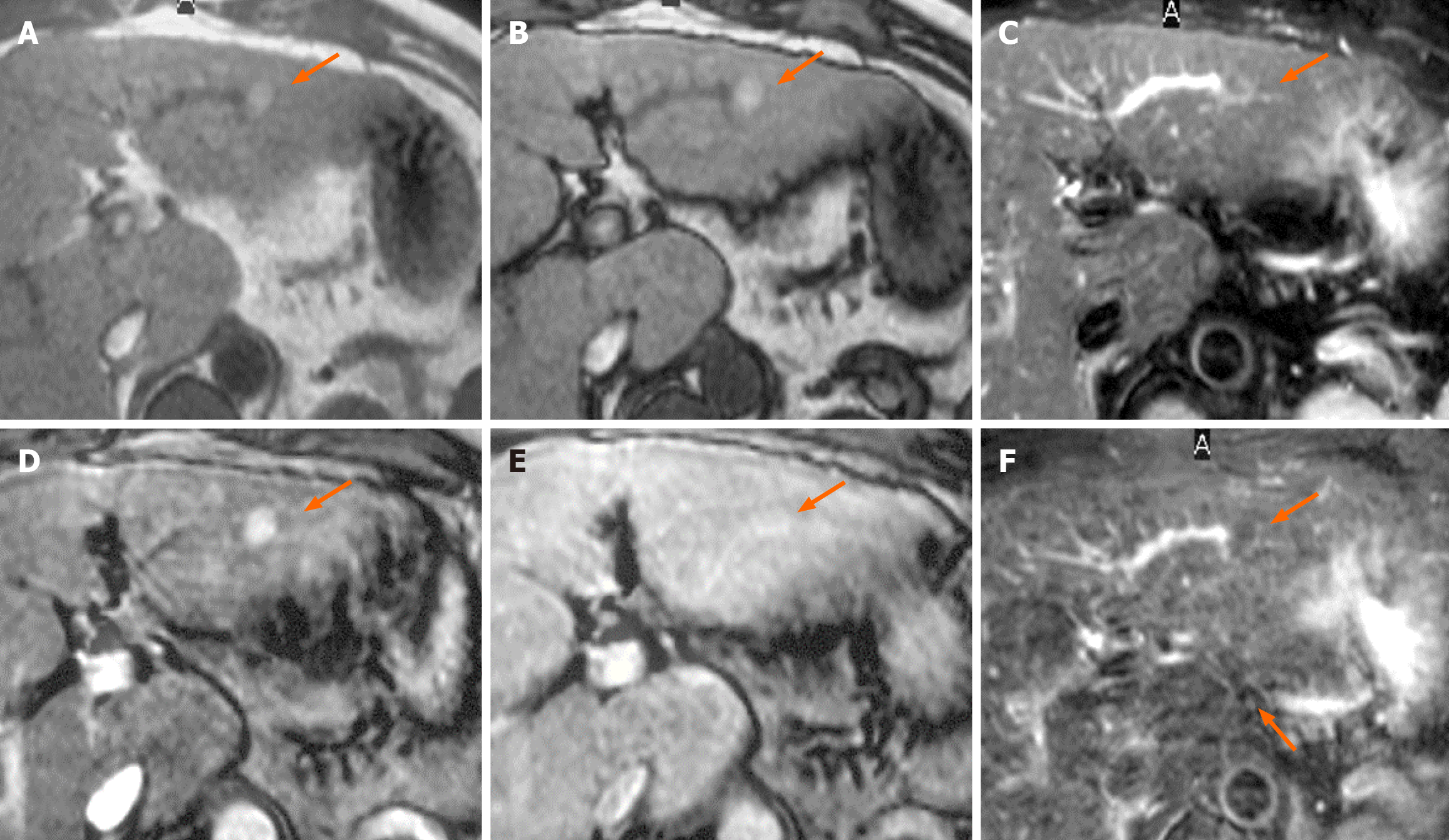
Table 2 presents the CT findings of the FNH-like lesions. Among the 13 patients with 26 FNH-like lesions, seven had a single lesion, while six had multiple lesions (two, one, and three patients had two, three, and over four lesions, respectively). The lesion size ranged from 10 mm to 30 mm (mean ± SD, 14.0 ± 5.5 mm). All 26 FNH-like lesions exhibited marked hypervascularity during the arterial phase on CE-CT and CTHA (seven patients) as well as washout appearance during the portal venous and equilibrium phases on CE-CT. Pathological examination revealed numerous unpaired arteries and sinusoidal capillarization in all patients, which were similar to those in HCC.
| Patient No. | No. of nodules | Size | Arterial enhancement pattern | Washout appearance | Modality | Central scar | Unpaired arteries and capillarization | Liver cell atypia | Scar-like fibrosis |
| 1 | 2 | 15 | Hyper | No | CECT/CTHA | - | + | - | - |
| 2 | 1 | 15 | Hyper | No | CECT/CTHA | - | + | - | - |
| 3 | > 4 | 10-15 | Hyper | No | CECT/CTHA | - | + | - | - |
| 4 | > 4 | 10-30 | Hyper | No | CECT/CTHA | + | + | - | + |
| 5 | 1 | 10 | Hyper | No | CECT | - | + | - | - |
| 6 | 3 | 10-30 | Hyper | No | CECT/CTHA | - | + | -/+ | - |
| 7 | 1 | 15 | Hyper | No | CECT/CTHA | + | + | - | + |
| 8 | 1 | 10 | Hyper | No | CECT | - | + | - | - |
| 9 | 1 | 10 | Hyper | No | CECT | - | + | - | - |
| 10 | > 4 | 10-15 | Hyper | No | CECT/CTHA | - | + | - | - |
| 11 | 2 | 15 | Hyper | No | CECT | - | + | - | - |
| 12 | 1 | 20 | Hyper | No | CECT | - | + | - | - |
| 13 | 1 | 14 | Hyper | No | CECT | + | + | - | - |
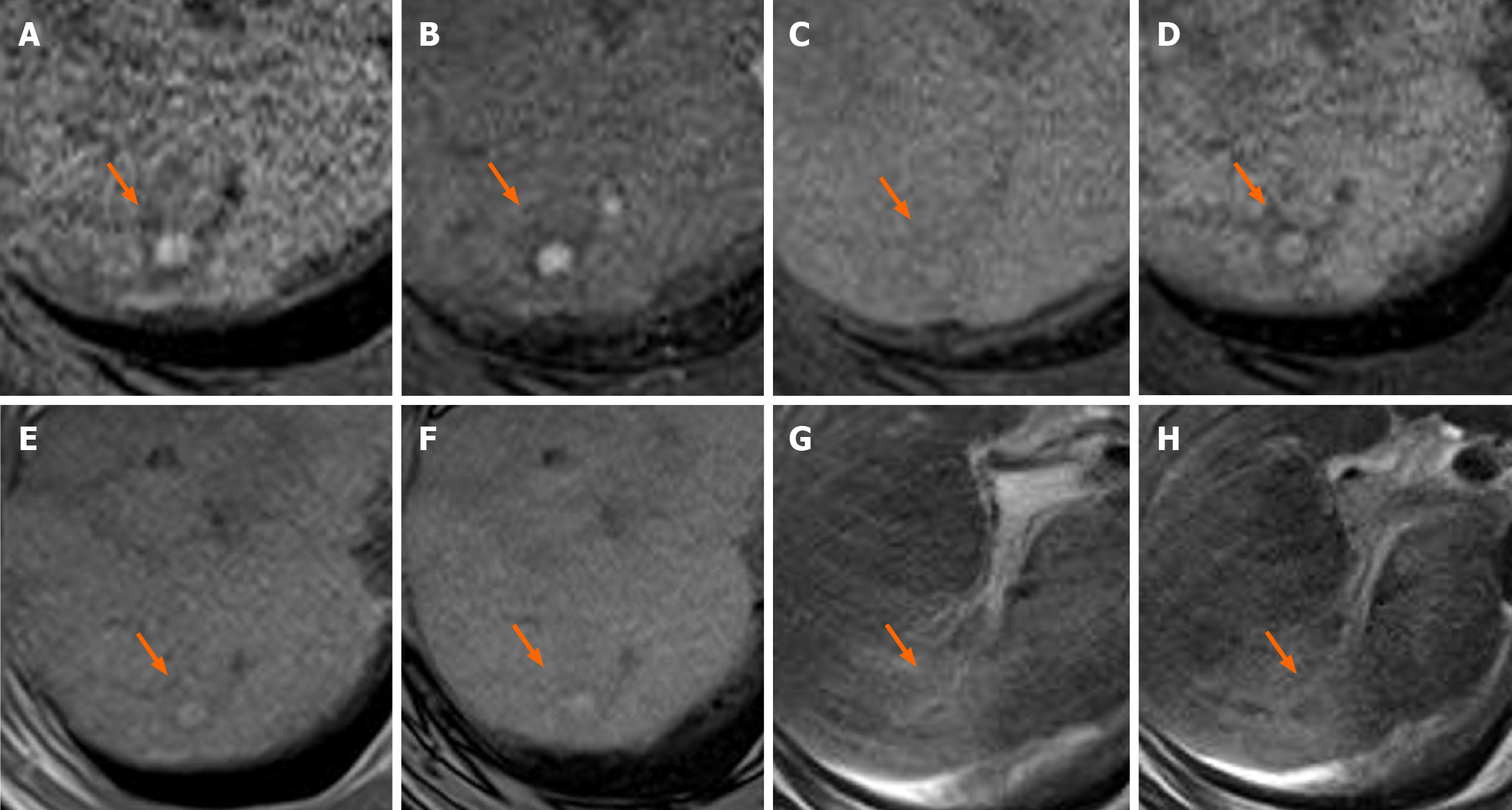
Table 3 presents the MRI findings of the FNH-like lesions. Among the 13 patients with FNH-like lesions, 11 (85%) showed high or iso-high intensity on T1WI, seven (54%) showed iso-high intensity on T2WI, six (46%) showed low intensity, three (23%) showed high-intensity central scars, and 10 (77%) showed no central scars on T2WI. Chemical shift artefacts were observed in all 13 patients with FNH-like lesions (Figures 1 and 2). In the six patients who underwent gadoxetic acid-enhanced MRI (Figures 3 and 4), all lesions exhibited marked homogeneous enhancement during the arterial phase. Two enhancement patterns were observed on the HBP: Heterogeneous hyperintense (n = 3, 43%) and ring-like enhancement (n = 4, 57%). In the eight patients who underwent SPIO-enhanced MRI, all lesions exhibited a marked homogeneous uptake pattern on post-SPIO-enhanced MRI (Figures 1 and 3).
| Patient No. | T1-weighted images | T2-weighted images | Central scar on magnetic resonance imaging | Chemical shift | Uptake pattern on the HBP | Uptake pattern on the SPIO |
| 1 | High | Iso-high | No | No | N/A | Homogenous |
| 2 | High | Iso-high | No | No | N/A | N/A |
| 3 | High | Low | No | No | N/A | Homogenous |
| 4 | Iso-high | Low | Yes | No | N/A | Homogenous |
| 5 | High | Iso-high | No | No | Ring | Homogenous |
| 6 | Low-high | Low | No | No | N/A | Homogenous |
| 7 | High | Iso | Yes | No | N/A | Homogenous |
| 8 | High | Low | No | No | Ring/hetero | Homogenous |
| 9 | High | Iso-high | No | No | N/A | Homogenous |
| 10 | High | Iso-high | No | No | Hetero | N/A |
| 11 | High | Low | No | No | Ring | N/A |
| 12 | Iso | Low | No | No | Hetero | N/A |
| 13 | Iso-high | Iso-high | Yes | No | Ring | N/A |
We studied pathologically confirmed FNH-like lesions in 13 patients with alcoholic cirrhosis. The radiological features of FNH-like lesions included hypervascularity, despite small lesion sizes, lack of a “corona-like” enhancement in the late phase on CTHA, high-intensity on T1WI, slightly high- or iso-intensity on T2WI, no signal decrease in out-of-phase images, and complete SPIO uptake or incomplete/partial uptake of gadoxetic acid. There have been several recent reports of FNH-like lesions in cirrhotic livers[1-6]. Although there is currently no established definition for FNH-like lesions/nodules, FNH is known to develop in normal livers. Accordingly, FNH-like lesions refer to similar nodules observed in patients with chronic liver disease[7]. These lesions have been extensively reported in alcoholic liver disease[8-10]. Most of these hyperplastic lesions are highly vascular and relatively small; additionally, they are difficult to differentiate from HCC lesions, especially early-stage HCC lesions[7-12]. We included 13 patients with alcoholic liver disease and hyperplastic liver lesions who were negative for hepatitis virus markers. Here, we discuss the imaging findings with respect to reported imaging characteristics of such lesions[13-20].
In our cases, except for a normal hepatic background and absence of a central scar, the pathological findings were consistent with those of FNH; accordingly, the patients were diagnosed with FNH-like lesions, based on the original text in the WHO classification (version 5)[9]. There has been no previous report on the radiographic findings of FNH-like lesions based on our exact definition since there remains no established definition. There were no differences in imaging diagnoses according to age or gender, as in previous reports. However, considering the similarities of the histopathology and imaging findings with those of FNH, it is highly likely that FNH-like lesions have been previously described as atypical FNH based on imaging findings. Approximately 80% of FNH cases are reported to be atypical on imaging[21]. Typical FNH on plain CT is characterized by hypo- to iso-attenuation, with homogeneous attenuation in early-phase contrast enhancement, and isoattenuation with the surrounding liver from the PVP to the equilibrium phase. On MRI, these lesions present as iso- to moderately hypointense on T1WIs, iso- to somewhat hyperintense on T2WIs, and iso- to somewhat hyperintense or ring-like hyperintense in the hepatocyte phase after gadoxetic acid-enhanced-MRI[13-22]. The central scar is generally hypointense on plain CT and early-phase contrast enhancement CT, with delayed enhancement into the equilibrium phase. Additionally, the central scar is hypointense on T1WIs and hyperintense on T2WIs; further, the region, including the surrounding area, has low uptake of gadoxetic acid during the hepatocyte phase. However, a central scar is only observed in approximately 50% of cases[23].
Single-level dynamic CTHA has indicated that blood flows from a dilated feeding artery inside the central scar; moreover, blood within the lesion directly flows into the hepatic vein via the fibrous septum and dilated veins at the margin of the lesion[24]. Grazioli et al[22] did not report the imaging characteristics of six nodules with hypointense enhancement; as such, whether there was a complete lack of enhancement currently remains unclear. Notably, the enhan
This study has several limitations. First, this retrospective study had inevitable selection bias. Additionally, all patients with FNH-like lesions were pathologically confirmed using fine-needle biopsy specimens rather than surgical specimens. Finally, there was no standard follow-up imaging of the FNH-like lesions; further, we did not describe the frequency of this phenomenon. Further large-scale multicenter studies using surgical specimens are warranted.
In conclusion, the present study showed that FNH-like lesions in chronic alcoholic liver disease demonstrate distinct imaging features, including small lesion size, hypervascularity, high signal on T1WIs, and characteristic uptake patterns on SPIO or gadoxetic acid-enhanced MRI. These features support a non-invasive differentiation from HCC, thereby reducing the need for invasive diagnostics and aiding appropriate clinical management. Overall, our findings provide valuable imaging criteria that can improve diagnostic accuracy and patient outcomes.
| 1. | Gluud C, Christoffersen P, Eriksen J, Wantzin P, Knudsen BB. Influence of ethanol on development of hyperplastic nodules in alcoholic men with micronodular cirrhosis. Gastroenterology. 1987;93:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Sugihara S, Nakashima O, Kiyomatsu K, Ijiri M, Edamitsu O, Kojiro M. A case of liver cirrhosis with a hyperplastic nodular lesion. Acta Pathol Jpn. 1990;40:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Terada T, Kitani S, Ueda K, Nakanuma Y, Kitagawa K, Masuda S. Adenomatous hyperplasia of the liver resembling focal nodular hyperplasia in patients with chronic liver disease. Virchows Arch A Pathol Anat Histopathol. 1993;422:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Quaglia A, Tibballs J, Grasso A, Prasad N, Nozza P, Davies SE, Burroughs AK, Watkinson A, Dhillon AP. Focal nodular hyperplasia-like areas in cirrhosis. Histopathology. 2003;42:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | An HJ, Illei P, Diflo T, John D, Morgan G, Teperman L, Theise N. Scirrhous changes in dysplastic nodules do not indicate high-grade status. J Gastroenterol Hepatol. 2003;18:660-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Nakashima O, Kurogi M, Yamaguchi R, Miyaaki H, Fujimoto M, Yano H, Kumabe T, Hayabuchi N, Hisatomi J, Sata M, Kojiro M. Unique hypervascular nodules in alcoholic liver cirrhosis: identical to focal nodular hyperplasia-like nodules? J Hepatol. 2004;41:992-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995;22:983-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 236] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2442] [Article Influence: 488.4] [Reference Citation Analysis (3)] |
| 10. | Lee YH, Kim SH, Cho MY, Shim KY, Kim MS. Focal nodular hyperplasia-like nodules in alcoholic liver cirrhosis: radiologic-pathologic correlation. AJR Am J Roentgenol. 2007;188:W459-W463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Choi JY, Lee HC, Yim JH, Shim JH, Lim YS, Shin YM, Yu ES, Suh DJ. Focal nodular hyperplasia or focal nodular hyperplasia-like lesions of the liver: a special emphasis on diagnosis. J Gastroenterol Hepatol. 2011;26:1004-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Yoneda N, Matsui O, Kitao A, Kita R, Kozaka K, Koda W, Kobayashi S, Gabata T, Ikeda H, Sato Y, Nakanuma Y. Hepatocyte transporter expression in FNH and FNH-like nodule: correlation with signal intensity on gadoxetic acid enhanced magnetic resonance images. Jpn J Radiol. 2012;30:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Miyaaki H, Ichikawa T, Taura N, Miuma S, Kawaguchi M, Kohno R, Isomoto H, Takeshima F, Nakashima O, Nakao K. Imaging of focal nodular hyperplastic-like nodules in alcoholic liver cirrhosis patients using gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid magnetic resonance imaging. Clin J Gastroenterol. 2011;4:266-272. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Vernuccio F, Gagliano DS, Cannella R, Ba-Ssalamah A, Tang A, Brancatelli G. Spectrum of liver lesions hyperintense on hepatobiliary phase: an approach by clinical setting. Insights Imaging. 2021;12:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Özcan HN, Karçaaltıncaba M, Seber T, Yalçın B, Oğuz B, Akyüz C, Haliloğlu M. Hepatocyte-specific contrast-enhanced MRI findings of focal nodular hyperplasia-like nodules in the liver following chemotherapy in pediatric cancer patients. Diagn Interv Radiol. 2020;26:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Fujiwara H, Sekine S, Onaya H, Shimada K, Mikata R, Arai Y. Ring-like enhancement of focal nodular hyperplasia with hepatobiliary-phase Gd-EOB-DTPA-enhanced magnetic resonance imaging: radiological-pathological correlation. Jpn J Radiol. 2011;29:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Mohajer K, Frydrychowicz A, Robbins JB, Loeffler AG, Reed TD, Reeder SB. Characterization of hepatic adenoma and focal nodular hyperplasia with gadoxetic acid. J Magn Reson Imaging. 2012;36:686-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Grieser C, Steffen IG, Kramme IB, Bläker H, Kilic E, Perez Fernandez CM, Seehofer D, Schott E, Hamm B, Denecke T. Gadoxetic acid enhanced MRI for differentiation of FNH and HCA: a single centre experience. Eur Radiol. 2014;24:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Kim JW, Lee CH, Kim SB, Park BN, Park YS, Lee J, Park CM. Washout appearance in Gd-EOB-DTPA-enhanced MR imaging: A differentiating feature between hepatocellular carcinoma with paradoxical uptake on the hepatobiliary phase and focal nodular hyperplasia-like nodules. J Magn Reson Imaging. 2017;45:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Murakami T, Tsurusaki M. Hypervascular benign and malignant liver tumors that require differentiation from hepatocellular carcinoma: key points of imaging diagnosis. Liver Cancer. 2014;3:85-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Ferlicot S, Kobeiter H, Tran Van Nhieu J, Cherqui D, Dhumeaux D, Mathieu D, Zafrani ES. MRI of atypical focal nodular hyperplasia of the liver: radiology-pathology correlation. AJR Am J Roentgenol. 2004;182:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Grazioli L, Bondioni MP, Haradome H, Motosugi U, Tinti R, Frittoli B, Gambarini S, Donato F, Colagrande S. Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology. 2012;262:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Nguyen BN, Fléjou JF, Terris B, Belghiti J, Degott C. Focal nodular hyperplasia of the liver: a comprehensive pathologic study of 305 lesions and recognition of new histologic forms. Am J Surg Pathol. 1999;23:1441-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 271] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Miyayama S, Matsui O, Ueda K, Kifune K, Yamashiro M, Yamamoto T, Komatsu T, Kumano T. Hemodynamics of small hepatic focal nodular hyperplasia: evaluation with single-level dynamic CT during hepatic arteriography. AJR Am J Roentgenol. 2000;174:1567-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |