Published online Jan 14, 2025. doi: 10.3748/wjg.v31.i2.100024
Revised: November 17, 2024
Accepted: November 22, 2024
Published online: January 14, 2025
Processing time: 134 Days and 17.6 Hours
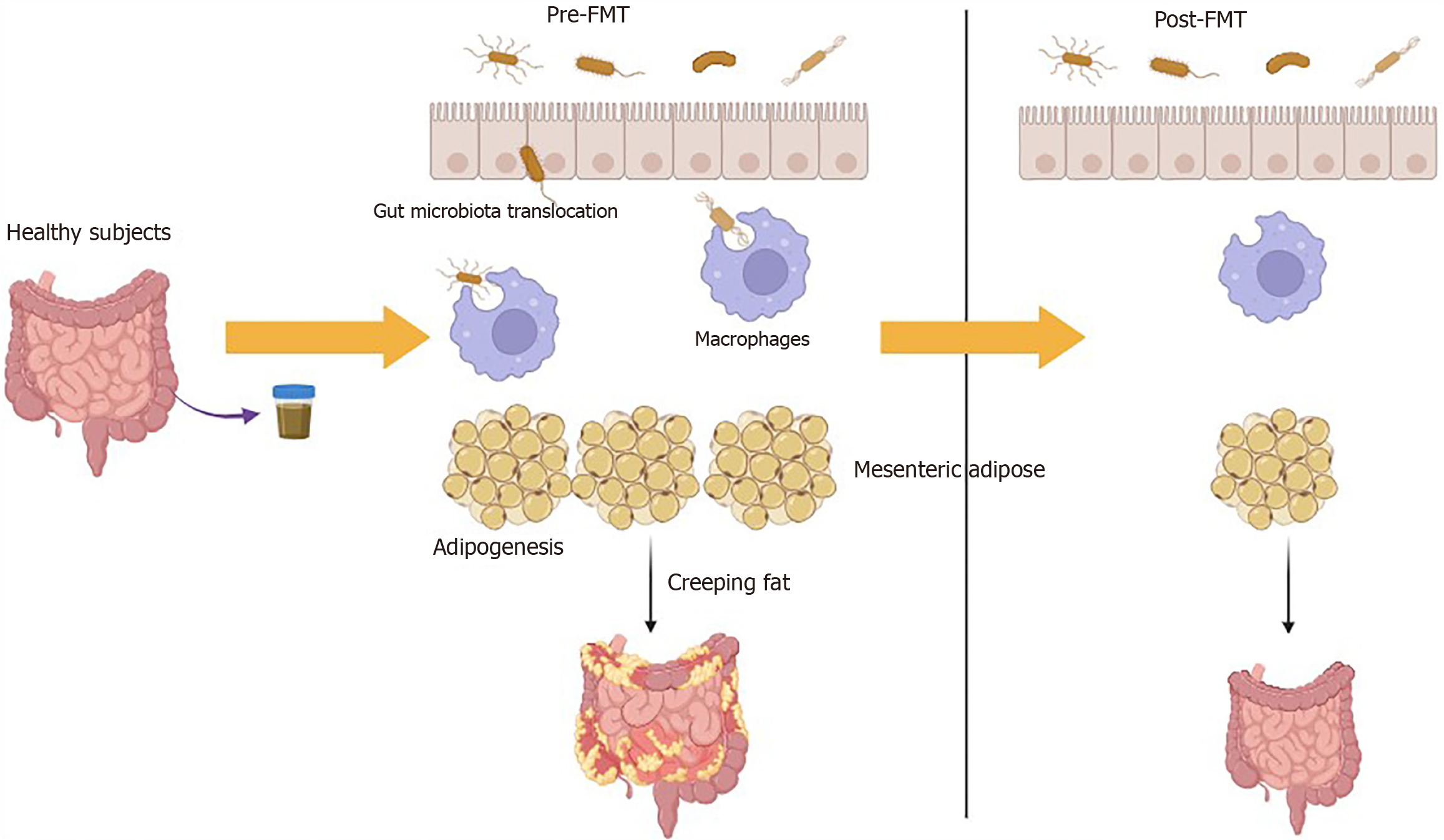
Inflammatory bowel disease, particularly Crohn's disease (CD), has been linked to modifications in mesenteric adipose tissue (MAT) and the phenomenon known as "creeping fat" (CrF). The presence of CrF is believed to serve as a predictor for early clinical recurrence following surgical intervention in patients with CD. Notably, the incorporation of the mesentery during ileocolic resection for CD has been correlated with a decrease in surgical recurrence, indicating the significant role of MAT in the pathogenesis of CD. While numerous studies have indicated that dysbiosis of the gut microbiota is a critical factor in the development of CD, the functional implications of translocated microbiota within the MAT of CD patients remain ambiguous. This manuscript commentary discusses a recent basic research conducted by Wu et al. In their study, intestinal bacteria from individuals were transplanted into CD model mice, revealing that fecal microbiota trans
Core Tip: Some studies have indicated that dysbiosis of the gut microbiota is a significant characteristic in the development of Crohn's disease (CD). However, the functional role of translocated microbiota in the mesenteric adipose tissue (MAT) of CD patients remains ambiguous. Evidence has shown that the translocation of viable microbiota into human MAT can polarize macrophages, leading to adipogenesis within the MAT and contributing to the formation of creeping fat (CrF) in individuals with CD. Nonetheless, it remains an important inquiry to elucidate the role of MAT-associated microbiota in the pathogenesis of CD. This manuscript aims to discuss the article by Wu et al, which explores the potential therapeutic value of fecal microbiota transplantation in the management of CD. Study by Wu et al suggested that the interactions among gut microbiota, MAT hypertrophy, and intestinal fibrosis may mutually reinforce one another in the pathogenesis of CD. Consequently, targeting MAT and CrF may hold promise as a therapeutic strategy for patients suffering from CD.
- Citation: Wang Y, Liu J. Interplay between creeping fat and gut microbiota: A brand-new perspective on fecal microbiota transplantation in Crohn's disease. World J Gastroenterol 2025; 31(2): 100024
- URL: https://www.wjgnet.com/1007-9327/full/v31/i2/100024.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i2.100024
Mesenteric adipose tissue (MAT) hypertrophy, commonly referred to as fat wrapping or creeping fat (CrF), is a characteristic feature of Crohn's disease (CD) and has been identified as a distinctive aspect of this condition[1]. The mesenteric mesoderm, which is encased by the intestinal endoderm, plays a crucial role in the development of the intestine through its contributions of cellular and connective tissue[2]. This pathobiological interaction persists into adulthood and is now recognized as a factor contributing to the pathological changes observed in CD. In cases of inflammation and intestinal strictures, CrF frequently envelops the affected intestine, and its presence correlates with the clinical activity and severity of inflammation associated with CD[3]. It is noteworthy that CrF predominantly occurs in the small intestine, particularly in the ileum, and is not observed in ulcerative colitis (UC), another type of inflammatory bowel disease[4]. Furthermore, CrF has been established as an anatomical marker that aids surgeons in delineating resection margins during surgical procedures. Additionally, it has been demonstrated that mesentery-based surgical approaches for CD are linked to enhanced long-term postoperative outcomes[5].
Numerous studies have indicated that dysbiosis of the gut microbiota is a significant characteristic in the development of CD[6]. However, the functional role of translocated microbiota in the MAT of CD patients remains ambiguous[7]. Additionally, human studies have shown a correlation between intestinal permeability and visceral adiposity[8], as well as metabolic syndrome in obese individuals[9]. A recent investigation has further substantiated the presence of bacteria and bacterial DNA in various MATs linked to the metabolic consequences of obesity[7]. Moreover, a study with a limited sample size identified a microbiotic signature at the phylogenetic level within the MAT of CD patients[10]. Notably, the translocation of viable microbiota into human MAT has been shown to polarize macrophages, which subsequently promotes adipogenesis in MAT and contributes to the development of CrF in CD patients[11]. Nonetheless, the precise role of MAT-associated microbiota in the pathogenesis of CD remains an important area for further exploration.
This manuscript examines the research conducted by Wu et al[12], focusing on the potential therapeutic benefits of fecal microbiota transplantation (FMT) in the management of CD. The findings of Wu et al[12] indicate that significant histopathological changes occur in the CrF and intestinal tissues of patients with CD. Additionally, there is a notable increase in the expression levels of pro-inflammatory cytokines within the CrF. Wu et al[12] suggested that the interplay between gut microbiota, MAT hypertrophy, and intestinal fibrosis might collectively contribute to the pathogenesis of CD. One method of modulating the microbiota is through FMT, which involves the transplantation of fecal microbiota from a healthy donor into the distal gastrointestinal tract of a patient. Notably, FMT was also employed by Wu et al[12], which demonstrated positive effects on body weight, colon length, and histopathological changes in mice treated with trinitrobenzenesulfonic acid. Additionally, FMT resulted in partial improvements in intestinal permeability, barrier function, serum levels of cytokines and adipokines, whereas FMT from CD patients exacerbated these parameters. FMT has been recognized as an effective therapeutic approach for Clostridium difficile infection[13] and is presently under investigation as a possible intervention for CD. In summary, Wu et al[12] emphasized the considerable promise of FMT in the management of CD.
MAT and CrF have been implicated in the pathogenesis of CD, with studies indicating that the ratio of intra-abdominal fat to total abdominal fat is elevated in individuals with CD compared to healthy controls. Additionally, a higher proportion of visceral fat has been linked to an increased incidence of postoperative disease recurrence[14,15]. CrF, characterized by its pathological alterations, is frequently observed in proximity to inflamed intestinal regions and is associated with heightened disease severity[16]. This type of fat exhibits distinct characteristics, such as increased size and enhanced immune cell infiltration, which differentiate it from normal mesenteric fat[17,18]. Moreover, MAT also functions as a reservoir for C-reactive protein and is susceptible to bacterial translocation, thereby providing further insight into the complex relationships between adipose tissue and inflammatory mechanisms in CD[19]. The recognition of this complex relationship has expanded the understanding of CD's pathogenesis, underscoring the significance of MAT and CrF in the context of inflammation, immune responses, and disease progression[20,21].
The inflammatory profile associated with MAT in patients diagnosed with CD is characterized by elevated levels of various cytokines, including tumour necrosis factor-α, interleukin (IL)-1β, and IL-6[22,23]. These elevated concentrations contribute to the inflammatory processes within the intestinal tract, while the downregulation of the protective adipokine adiponectin is implicated in the pathogenesis of CD. CrF have been identified as significant sources of pro-inflammatory and pro-fibrotic cytokines[24]. In the context of CD, the integrity of the intestinal barrier is compromised, facilitating the translocation of bacterial antigens and subsequently eliciting Th17 and Th1 immune responses[25]. The Th1 response is particularly characteristic of CD, leading to the secretion of cytokines such as IL-22, IL-1, interferon-γ, and IL-2, among others[26]. Notably, Th1 cells are more prevalent in CrF compared to the mucosal environment, which exhibits a higher infiltration of Th17 cells in response to bacterial infections. Furthermore, the CrF contains a greater proportion of M2 macrophages relative to M1 macrophages, in contrast to the lamina propria, where M1 macrophages are more predo
The compromised integrity of the intestinal barrier facilitates the translocation of gut-derived bacteria (Figure 1). Research indicates that up to 27% of patients with CD experience bacterial translocation to mesenteric fat, in contrast to 13% of healthy controls. This phenomenon has also been observed in models of experimental colitis and ileitis[19]. CD is associated with a microbiome profile characterized by an increased presence of Proteobacteria and Clostridium innocuum, with the relative abundance of these bacteria being influenced by the clinical status of the disease[10,11]. Furthermore, lymphatic flow is crucial for the transport of bacterial antigens and immune cells[30]. One hypothesis regarding lymphatic vessels suggests that CrF may be exacerbated by the leakage of fatty chylomicrons into the mesentery, facilitated by highly permeable lymphatic vessels[31,32]. Improving the integrity and pumping function of lymphatic vessels could potentially mitigate inflammation in MAT, thereby supporting the notion that the leakage of antigens triggers inflammatory responses and adipogenesis in MAT[33]. Indeed, single-cell RNA sequencing has identified CrF as both pro-fibrotic and pro-adipogenic, characterized by a diverse array of activated immune cells responding to microbial stimuli[11].
The existing literature on FMT for CD is relatively limited compared to that for UC. Case reports have yielded mixed outcomes, with some indicating both clinical and endoscopic remission, while others reported no significant effects[34]. Notably, a case involving a patient with severe, complicated CD documented a successful response to FMT[35]. In a cohort study involving 30 patients with refractory mid-gut CD, a single FMT administered via the nasoduodenal route resulted in a 77% rate of clinical remission one month post-treatment[36]. Furthermore, FMT may represent a viable treatment option for pediatric CD, as a recent case series reported remission in 5 out of 9 patients (56%) following FMT, with 7 out of 9 patients (78%) exhibiting engraftment of donor microbiota[37]. Previous investigations into FMT for active CD have produced inconsistent results. Among 20 subjects enrolled in an FMT study, 19 provided complete follow-up data. Although most participants experienced improvement post-FMT, the clinical outcomes varied significantly, with one individual who had severe disease prior to FMT ultimately requiring colectomy afterward[38]. Conversely, a recent meta-analysis indicated that FMT significantly decreased CD activity index scores within 4 weeks to 8 weeks following the procedure[39]. Collectively, these findings suggest that FMT may hold potential as a therapeutic approach for CD. However, further research is essential to evaluate both its clinical efficacy and the associated alterations in the gut microbiota of affected patients. In the study conducted by Wu et al[12], the transplantation of intestinal bacteria from individuals into CD model mice revealed that FMT from healthy donors alleviated CD symptoms, whereas FMT from CD patients exacerbated these symptoms. Importantly, FMT was found to influence intestinal permeability, barrier function, and levels of pro-inflammatory factors and adipokines. These results imply that targeting MAT and CrF may provide promising avenues for therapeutic interventions in CD patients. Consequently, the gut microbiota appears to play a pivotal role in the histopathology of CD, and strategies aimed at targeting MAT and CrF may be beneficial in the treatment of CD.
Recent investigations have documented specific alterations in the gut microbiota of recipients following FMT. A study indicated a significant increase in alpha diversity post-FMT, in contrast to sham group. However, this enhancement was transient, with alpha diversity reverting to baseline levels 14 weeks after FMT[34]. Similarly, research conducted by Vaughn et al[38] suggested that a single colonoscopic FMT could induce short-term modifications in the fecal bacterial composition of patients with active CD, thereby increasing the diversity of the intestinal microbiota. Another study revealed that there was an increase in the abundance of Collinsella and several genera within the Lachnospiraceae family in FMT-responders. Nonetheless, certain genera from the Ruminococcaceae and Lachnospiraceae families remained underrepresented in post-FMT responders, and some members of the Enterobacteriaceae family persisted in two of the responders. Post-FMT, specific members of Lachnospiraceae were found to be more prevalent in responders, while Ruminococcaceae
Overall, the gut microbiota plays a crucial role in the histopathology of CD, and thus, targeting MAT and CrF may represent a promising avenue for treatment in this patient population.
We thanks to Wang Y (Division of Digestive Endoscopy, Department of General Surgery, The First Affiliated Hospital with Nanjing Medical University) for his support in revising this letter.
| 1. | Hwang N, Kang D, Shin SJ, Yoon BK, Chun J, Kim JW, Fang S. Creeping fat exhibits distinct Inflammation-specific adipogenic preadipocytes in Crohn's disease. Front Immunol. 2023;14:1198905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Byrnes KG, Walsh D, Dockery P, McDermott K, Coffey JC. Anatomy of the mesentery: Current understanding and mechanisms of attachment. Semin Cell Dev Biol. 2019;92:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Feng Q, Xu XT, Zhou Y, Yan YQ, Ran ZH, Zhu J. Creeping fat in patients with ileo-colonic Crohn's disease correlates with disease activity and severity of inflammation: A preliminary study using energy spectral computed tomography. J Dig Dis. 2018;19:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol. 2014;5:462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Coffey CJ, Kiernan MG, Sahebally SM, Jarrar A, Burke JP, Kiely PA, Shen B, Waldron D, Peirce C, Moloney M, Skelly M, Tibbitts P, Hidayat H, Faul PN, Healy V, O'Leary PD, Walsh LG, Dockery P, O'Connell RP, Martin ST, Shanahan F, Fiocchi C, Dunne CP. Inclusion of the Mesentery in Ileocolic Resection for Crohn's Disease is Associated With Reduced Surgical Recurrence. J Crohns Colitis. 2018;12:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 6. | Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell. 2019;178:1041-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 7. | Cai J, Sun L, Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe. 2022;30:289-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 429] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 8. | Gummesson A, Carlsson LM, Storlien LH, Bäckhed F, Lundin P, Löfgren L, Stenlöf K, Lam YY, Fagerberg B, Carlsson B. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring). 2011;19:2280-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Teixeira TF, Souza NC, Chiarello PG, Franceschini SC, Bressan J, Ferreira CL, Peluzio Mdo C. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 10. | Serena C, Queipo-Ortuño M, Millan M, Sanchez-Alcoholado L, Caro A, Espina B, Menacho M, Bautista M, Monfort-Ferré D, Terrón-Puig M, Núñez-Roa C, Maymó-Masip E, Rodriguez MM, Tinahones FJ, Espin E, Martí M, Fernández-Veledo S, Vendrell J. Microbial Signature in Adipose Tissue of Crohn's Disease Patients. J Clin Med. 2020;9:2448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 11. | Ha CWY, Martin A, Sepich-Poore GD, Shi B, Wang Y, Gouin K, Humphrey G, Sanders K, Ratnayake Y, Chan KSL, Hendrick G, Caldera JR, Arias C, Moskowitz JE, Ho Sui SJ, Yang S, Underhill D, Brady MJ, Knott S, Kaihara K, Steinbaugh MJ, Li H, McGovern DPB, Knight R, Fleshner P, Devkota S. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell. 2020;183:666-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 12. | Wu Q, Yuan LW, Yang LC, Zhang YW, Yao HC, Peng LX, Yao BJ, Jiang ZX. Role of gut microbiota in Crohn's disease pathogenesis: Insights from fecal microbiota transplantation in mouse model. World J Gastroenterol. 2024;30:3689-3704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (2)] |
| 13. | Khoruts A, Staley C, Sadowsky MJ. Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol. 2021;18:67-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 14. | Kredel LI, Batra A, Stroh T, Kühl AA, Zeitz M, Erben U, Siegmund B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn's disease. Gut. 2013;62:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Yang L, Liu G, Zhang Y, Yao B, Wu Q, Peng L, Wang X, Yuan L. Quantitative analysis of adipose tissue for predicting Crohn's disease postoperative endoscopic recurrence and anastomotic ulcer. Int J Colorectal Dis. 2023;38:170. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Mao R, Kurada S, Gordon IO, Baker ME, Gandhi N, McDonald C, Coffey JC, Rieder F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn's Disease. Inflamm Bowel Dis. 2019;25:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 17. | Peyrin-Biroulet L, Chamaillard M, Gonzalez F, Beclin E, Decourcelle C, Antunes L, Gay J, Neut C, Colombel JF, Desreumaux P. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 18. | Westcott ED, Mattacks CA, Windsor AC, Knight SC, Pond CM. Perinodal adipose tissue and fatty acid composition of lymphoid tissues in patients with and without Crohn's disease and their implications for the etiology and treatment of CD. Ann N Y Acad Sci. 2006;1072:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C, Colombel JF, Desreumaux P. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61:78-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Bilski J, Mazur-Bialy AI, Wierdak M, Brzozowski T. The impact of physical activity and nutrition on inflammatory bowel disease: the potential role of cross talk between adipose tissue and skeletal muscle. J Physiol Pharmacol. 2013;64:143-155. [PubMed] |
| 21. | Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut. 2012;61:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 22. | Yamamoto K, Kiyohara T, Murayama Y, Kihara S, Okamoto Y, Funahashi T, Ito T, Nezu R, Tsutsui S, Miyagawa JI, Tamura S, Matsuzawa Y, Shimomura I, Shinomura Y. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005;54:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules. 2019;9:780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Guedj K, Abitbol Y, Cazals-Hatem D, Morvan M, Maggiori L, Panis Y, Bouhnik Y, Caligiuri G, Corcos O, Nicoletti A. Adipocytes orchestrate the formation of tertiary lymphoid organs in the creeping fat of Crohn's disease affected mesentery. J Autoimmun. 2019;103:102281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn's disease. Immunol Rev. 2005;206:277-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 7.5] [Reference Citation Analysis (2)] |
| 26. | Li N, Shi RH. Updated review on immune factors in pathogenesis of Crohn's disease. World J Gastroenterol. 2018;24:15-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (6)] |
| 27. | Kredel LI, Jödicke LJ, Scheffold A, Gröne J, Glauben R, Erben U, Kühl AA, Siegmund B. T-cell Composition in Ileal and Colonic Creeping Fat - Separating Ileal from Colonic Crohn's Disease. J Crohns Colitis. 2019;13:79-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Lissner D, Schumann M, Batra A, Kredel LI, Kühl AA, Erben U, May C, Schulzke JD, Siegmund B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 29. | Dharmasiri S, Garrido-Martin EM, Harris RJ, Bateman AC, Collins JE, Cummings JRF, Sanchez-Elsner T. Human Intestinal Macrophages Are Involved in the Pathology of Both Ulcerative Colitis and Crohn Disease. Inflamm Bowel Dis. 2021;27:1641-1652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 30. | Dongaonkar RM, Nguyen TL, Quick CM, Hardy J, Laine GA, Wilson E, Stewart RH. Adaptation of mesenteric lymphatic vessels to prolonged changes in transmural pressure. Am J Physiol Heart Circ Physiol. 2013;305:H203-H210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. 2014;124:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 32. | Randolph GJ, Bala S, Rahier JF, Johnson MW, Wang PL, Nalbantoglu I, Dubuquoy L, Chau A, Pariente B, Kartheuser A, Zinselmeyer BH, Colombel JF. Lymphoid Aggregates Remodel Lymphatic Collecting Vessels that Serve Mesenteric Lymph Nodes in Crohn Disease. Am J Pathol. 2016;186:3066-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Yin Y, Yang J, Pan Y, Guo Z, Gao Y, Huang L, Zhou D, Ge Y, Guo F, Zhu W, Song Y, Li Y. Chylomicrons-Simulating Sustained Drug Release in Mesenteric Lymphatics for the Treatment of Crohn's-Like Colitis. J Crohns Colitis. 2021;15:631-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, Bourrier A, Le Gall G, Lalande V, De Rougemont A, Kirchgesner J, Daguenel A, Cachanado M, Rousseau A, Drouet É, Rosenzwajg M, Hagege H, Dray X, Klatzman D, Marteau P; Saint-Antoine IBD Network, Beaugerie L, Simon T. Fecal microbiota transplantation to maintain remission in Crohn's disease: a pilot randomized controlled study. Microbiome. 2020;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (1)] |
| 35. | Zhang FM, Wang HG, Wang M, Cui BT, Fan ZN, Ji GZ. Fecal microbiota transplantation for severe enterocolonic fistulizing Crohn's disease. World J Gastroenterol. 2013;19:7213-7216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (1)] |
| 36. | Cui B, Feng Q, Wang H, Wang M, Peng Z, Li P, Huang G, Liu Z, Wu P, Fan Z, Ji G, Wang X, Wu K, Fan D, Zhang F. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: safety, feasibility, and efficacy trial results. J Gastroenterol Hepatol. 2015;30:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 37. | Suskind DL, Brittnacher MJ, Wahbeh G, Shaffer ML, Hayden HS, Qin X, Singh N, Damman CJ, Hager KR, Nielson H, Miller SI. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn's disease. Inflamm Bowel Dis. 2015;21:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 38. | Vaughn BP, Vatanen T, Allegretti JR, Bai A, Xavier RJ, Korzenik J, Gevers D, Ting A, Robson SC, Moss AC. Increased Intestinal Microbial Diversity Following Fecal Microbiota Transplant for Active Crohn's Disease. Inflamm Bowel Dis. 2016;22:2182-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 39. | Zhou S, Cui Y, Zhang Y, Zhao T, Cong J. Fecal microbiota transplantation for induction of remission in Crohn's disease: a systematic review and meta-analysis. Int J Colorectal Dis. 2023;38:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Gutin L, Piceno Y, Fadrosh D, Lynch K, Zydek M, Kassam Z, LaMere B, Terdiman J, Ma A, Somsouk M, Lynch S, El-Nachef N. Fecal microbiota transplant for Crohn disease: A study evaluating safety, efficacy, and microbiome profile. United European Gastroenterol J. 2019;7:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 41. | Papa E, Docktor M, Smillie C, Weber S, Preheim SP, Gevers D, Giannoukos G, Ciulla D, Tabbaa D, Ingram J, Schauer DB, Ward DV, Korzenik JR, Xavier RJ, Bousvaros A, Alm EJ. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One. 2012;7:e39242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 42. | Mondot S, Kang S, Furet JP, Aguirre de Carcer D, McSweeney C, Morrison M, Marteau P, Doré J, Leclerc M. Highlighting new phylogenetic specificities of Crohn's disease microbiota. Inflamm Bowel Dis. 2011;17:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |