INTRODUCTION
The liver, a complex organ with multifaceted functions, operates under rigorous physiological regulation. As individuals age, the liver experiences substantial alterations in its composition, functionality, and vulnerability to diseases[1,2]. Currently, hepatic disorders contribute significantly to global morbidity and mortality. Previous research indicates that the buildup of age-related damage activates compensatory pathways in liver cells, such as cellular senescence and altered nutrient sensing. Excessive activation of these pathways can hinder liver regeneration and exacerbate liver disease progression[3]. For instance, aging can lead to reduced liver perfusion, impaired hepatocyte function, and altered microcirculation within the liver sinusoids. These alterations have a significant negative impact on liver function, increasing the risk of liver diseases and complications in the elderly[4]. Lifestyle modifications have demonstrated efficacy in treating certain prevalent liver diseases, highlighting the crucial role of lifestyle in disease outcomes[5]. These interventions primarily encompass caloric restriction (CR) and physical exercise. CR is recognized as an effective dietary strategy that enhances health and prolongs the lifespan of species[6-8]. Exercise stimulates the release of beneficial substances from muscles, contributing to overall health and preventing metabolic disorders[9,10]. Given its pivotal regulatory functions, the aging liver exhibits heightened susceptibility to disease and disrupts overall metabolic homeostasis. A bibliometric analysis has revealed an exponential surge in the number of publications concerning hepatic aging over the past four decades[11]. The objective of this article is to delineate the critical facets of liver aging, synthesize the latest research on lifestyle interventions, and underscore their potential to improve the quality of life for individuals entering and progressing through middle age and beyond[12,13].
BASIC FUNCTIONS OF THE LIVER
The liver plays a critical role in various physiological functions. Following food consumption, it metabolizes nutrients through various biochemical reactions. For instance, the body’s decision to store glucose as glycogen or produce it through gluconeogenesis is determined by the level of energy intake. Moreover, the liver metabolizes proteins, fats, vitamins, hormones, and other chemicals. Beyond its metabolic functions, it also regulates blood volume, supports immune responses, decomposes xenobiotics, and modulates endocrine activities. These processes are essential for maintaining the organism’s stability and homeostasis[1].
CHANGES IN THE LIVER WITH AGING
Aging is associated with numerous structural and functional modifications in the liver. Notable alterations include a decline in liver volume and blood flow, an accumulation of lipofuscin in hepatocytes, a decrease in the density of smooth endoplasmic reticulum, compromised bile acid secretion, and reduced capacity for liver regeneration[14,15]. These age-related physiological modifications might amplify liver damage and inflammation, thereby heightening the risk of chronic liver disease and mortality[16]. For instance, analysis of multiple external datasets has uncovered a significant overexpression of 40 genes from the HepG2 senescence signature in various conditions, including chronic liver disease. This finding offers compelling evidence for the occurrence of hepatocyte senescence in chronic liver disease and suggests that these genes may serve as potential biomarkers for the disease’s progression[17].
MOLECULAR MECHANISMS OF LIVER AGING
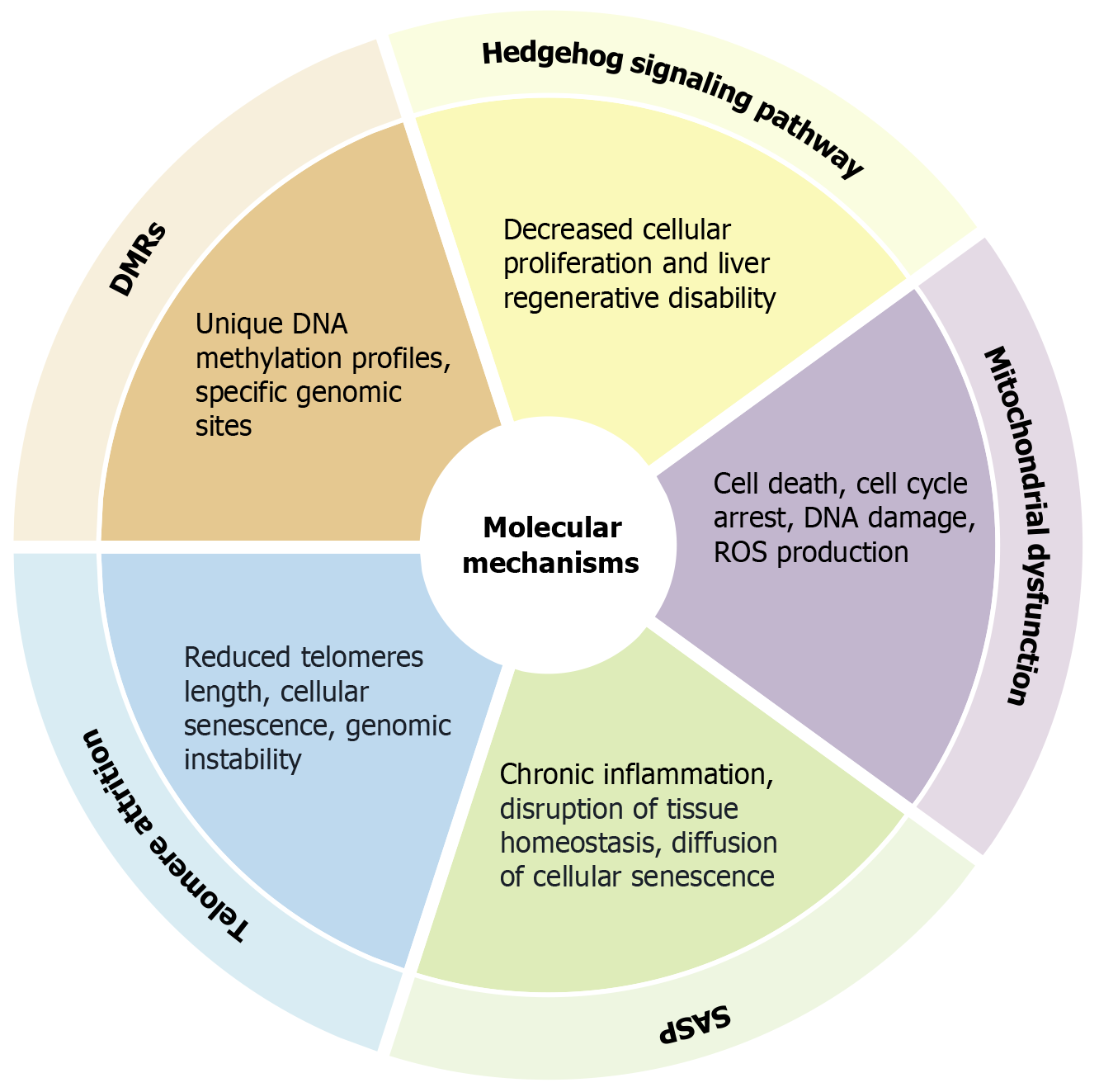
Liver aging encompasses intricate processes resulting in the accumulation of senescent cells within the hepatic tissue. This accumulation is influenced by both intrinsic aging processes and extrinsic elements, including habits, environmental exposures, and chronic liver pathologies. Hepatocyte senescence is a complex phenomenon involving interwoven signaling pathways. The hedgehog signaling system is an important biochemical route that coordinates many developmental processes while maintaining tissue homeostasis. It is essential in regulating cell survival, proliferation, and differentiation. This pathway is critical for developing and maintaining hepatocytes and bile ducts within the liver. The hedgehog signaling pathway initiates the activation of yes-associated protein 1 (Yap1) in hepatic stem cells, thereby promoting their differentiation into myofibroblasts. Simultaneously, myofibroblasts facilitate Yap1 activation in hepatocytes through paracrine signaling, which in turn stimulates hepatocyte proliferation and dedifferentiation, thereby contributing to liver regeneration. The interplay between the hedgehog pathway and Yap1 in liver regeneration presents promising therapeutic targets for the treatment of cirrhosis and hepatocellular carcinoma[18]. This regulator, which plays a crucial role in controlling mitosis and morphogenesis, is inhibited in senescent hepatocytes, which reduces cellular proliferation and impairs the liver’s ability to regenerate following injury. Additionally, disruption of this pathway in young hepatocytes recapitulates the aging process, thereby underscoring its essential role in maintaining hepatocyte viability[19].
Furthermore, mitochondria, essential organelles within cells, are primary producers of intracellular reactive oxygen species (ROS) and are particularly prone to oxidative stress. ROS, unstable oxygen derivatives, can harm cells and tissues when present in large quantities. Oxidative damage to mitochondria can impair their functionality, leading to cell death via apoptosis and necrosis. Mitochondrial dysfunction often intensifies ROS production, creating a harmful cycle[20]. Studies have demonstrated that senescent cells manifest distinct characteristics, including cell cycle arrest, DNA damage, elevated production of ROS, and the secretion of a senescence-associated secretory phenotype (SASP). Pro-inflammatory cytokines, chemokines, growth factors, and proteases are released by senescent cells in SASP. SASP includes a range of secreted factors and signaling molecules released by senescent cells. These substances can alter the microenvironment and impact nearby cells. In response to oxidative stress or chronic DNA damage, SASP first aids in tissue healing and encourages the immune system to eliminate damaged cells[21]. However, long-term SASP activation causes chronic inflammation, throws off tissue homeostasis, spreads senescence to nearby cells, and fuels aging-related diseases like cancer, fibrosis, and neurodegeneration. These attributes can perturb hepatic function and facilitate the advancement of liver disease[22-24].
Differentially methylated regions denote specific genomic sites where methylation of DNA takes place. These regions are critical for the regulation of gene expression, as the methylation status of these sites modifies chromatin structure, thereby influencing the accessibility of transcription factors to the DNA. Epigenetic alterations observed in aging and regenerating livers exhibit unique DNA methylation profiles in most differentially methylated regions located within gene structures and repetitive sequences[25]. Telomere attrition is a contributing factor to the aging process. During each cell division cycle, telomeres progressively shorten due to DNA polymerase’s inability to replicate the terminal segments of chromosomes fully[26]. When telomeres reach a critically reduced length, they lose their capacity to preserve cellular integrity, leading to cellular senescence or genomic instability. The gradual build-up of senescent cells throughout life is linked to the secretion of pro-inflammatory cytokines, which initiate age-related diseases and degenerative conditions[26-29]. The molecular mechanisms involved in the aging process of the liver are summarized in Figure 1.
Figure 1 Summary of molecular mechanisms in liver aging.
Cellular senescence is linked to changes and effects at the molecular level. These include the role of the Hedgehog signaling pathway in inhibiting liver regeneration and cellular proliferation, the effects of mitochondrial dysfunction, such as reactive oxygen species production and apoptosis, the link between senescence-associated secretory phenotype and the spread of senescence and chronic inflammation, the traits of telomere attrition that cause genomic instability, and the distinct DNA methylation profiles in differentially methylated regions. DMRs: Differentially methylated regions; ROS: Reactive oxygen species; SASP: Senescence-associated secretory phenotype.
Nevertheless, these molecular mechanisms are not independent entities; rather, they are interrelated and exhibit mutual influence. For instance, the p53 and DNA damage response pathways are activated in response to telomere dysfunction-induced DNA damage. This activation leads to the suppression of peroxisome proliferator-activated receptor-gamma coactivator-1α and peroxisome proliferator-activated receptor-gamma coactivator-1β, culminating in mitochondrial dysfunction[30]. The hedgehog signaling pathway regulates the expression and activity of telomerase reverse transcriptase (hTERT) via its critical transcription factors, GLI1 and GLI2. These factors directly interact with the hTERT promoter region, thereby enhancing telomerase activity and promoting hTERT transcription. Telomerase replenishes telomeric repeats to prevent shortening and maintain telomere length[31].
INTERVENTIONS OF LIFESTYLE
One of the most fascinating and challenging areas of scientific study is anti-aging treatments. Presently, mitigating liver aging encompasses CR, moderate physical exercise, and using botanical compounds for therapeutic purposes[32]. CR induces metabolic stress, eliciting adaptive cellular responses that augment repair mechanisms, diminish inflammation, and enhance insulin sensitivity. These alterations are conducive to the prevention of age-related pathologies, as CR has been linked to improvements in mitochondrial function, maintenance of telomere integrity, and suppression of the SASP, potentially mitigating age-related cellular damage and promoting hepatic health. Notably, the dietary composition is instrumental in this context. Diets abundant in fruits, vegetables, whole grains, lean proteins, and healthful lipids, such as the Mediterranean diet, confer additional health advantages by supplying antioxidants, phytochemicals, and anti-inflammatory agents. The synergistic application of CR with a nutrient-rich dietary regimen shows significant potential in fostering longevity and promoting healthy aging processes[7,8,33]. A study implemented a 30% CR in excision repair cross-complementation group 1-deficient mice to evaluate its effects on lifespan and liver aging. Histological examination indicated that CR diminished hepatocyte nuclear anomalies, such as polyploidy, and decreased H2AX levels. The findings suggest that CR alleviates age-related liver pathologies and may serve as a potential therapeutic approach for mitigating age-associated liver deterioration[34]. Long-term CR was shown to decelerate the pace of aging in healthy adults, as measured by the DunedinPACE[35] DNA methylation algorithm. Participants in the CR group exhibited a significant reduction in DunedinPACE values by 0.29 units (95% confidence interval: -0.45, -0.13) at 12 months and 0.25 units (95% confidence interval: -0.41, -0.09) at 24 months compared to the control group. These reductions correspond to a 2-3% slower pace of aging, highlighting the potential of CR as a promising intervention to enhance health and promote longevity[36].
Physical exercise is acknowledged as a fundamental component of a healthy lifestyle, capable of preventing chronic diseases such as cardiovascular and metabolic disorders, as well as cognitive deterioration. Research suggests that myokines, secreted by the activated skeletal muscles during physical activity, likely act as mediators that impart these health advantages. They enhance metabolic functions, increase glucose absorption, stimulate fat combustion, and modulate the regeneration of skeletal muscle tissue[10,37]. Resistance exercise has been demonstrated to enhance muscle strength among older adults significantly. A recent study implemented a long-term treadmill exercise regimen in transgenic mice. Histopathological evaluation through hematoxylin and eosin staining and CD68 immunofluorescence revealed significant changes in liver tissue. Notably, exercise led to a more organized arrangement of hepatocytes, the normalization of hepatic sinusoids, and a marked reduction in Kupffer cell aggregation. These enhancements indicate that exercise has a notable protective effect against liver aging, underlining its potential as a therapeutic intervention for age-related liver disease[38]. Moreover, the synergistic effect of exercise combined with dietary interventions has been demonstrated to be more efficacious in retarding liver aging than either intervention administered independently.
Diets abundant in health-beneficial botanical compounds, such as anthocyanidins, flavones, and isoflavones, have been implicated in the postponement of liver aging[39]. Anthocyanins are renowned for their potent antioxidant properties. Individuals with a higher intake of dietary anthocyanins exhibit a slower progression of aging-related characteristics[40,41]. Paeonol effectively neutralizes free radicals and modulates the activity of antioxidant enzymes. It significantly improves cognitive impairments and attenuates neuropathological changes in mouse models[42]. Similarly, silymarin is efficacious in mitigating D-galactose-induced mitochondrial dysfunction and demonstrates a protective role in liver health[43,44]. Artificial intelligence (AI) and machine learning (ML) are being used more and more in medicine in the digital age, especially in the treatment of liver illnesses. Computer imaging driven by AI and ML improves diagnostic precision, automates segmentation, and makes minimally invasive surgery and preoperative planning easier[45]. However, precisely defining tumor borders in intricate situations continues to be difficult. Despite this, the continuous development of technology gives hope that AI and ML will continue to make important strides, benefiting patients even more and enhancing the effectiveness of healthcare services[46]. Aging is an inevitable aspect of life that demands scientific scrutiny. Despite its complexity, recent advancements in algorithmic development have significantly propelled aging research forward. The field stands to benefit immensely from advanced data analytical techniques and accelerated drug discovery processes, particularly within personalized medicine frameworks. Quantum algorithms, such as the quantum approximate optimization algorithm, demonstrate the potential to outperform classical methods in solving complex optimization problems. This could revolutionize our understanding of aging mechanisms and pave the way for innovative therapeutic strategies[47].
CONCLUSION
Aging is a complex process involving various changes that increase susceptibility to chronic illnesses. Advancing age is not only a risk factor for the initiation of chronic liver conditions but also a primary driver of disease progression and the transition to end-stage liver disease. Understanding the molecular mechanisms underlying aging could facilitate the identification of therapeutic targets for age-related diseases and decelerate the aging process. Interleukin-6 (IL-6) and IL-8, key components of SASP, are implicated in aging, chronic inflammation, and age-related pathologies. These cytokines represent well-established therapeutic targets, with rapid clinical trial progression. Tocilizumab, an IL-6 receptor antagonist, inhibits signaling pathways critical for triple-negative breast cancer growth and angiogenesis. This mechanism positions Tocilizumab as a potential therapeutic strategy for triple-negative breast cancer, particularly in suppressing tumor-associated angiogenesis[48]. Therapeutic strategies targeting these cytokines may open new avenues for anti-aging interventions. Further research is essential to delineate their mechanisms of action in specific diseases and to facilitate the development of targeted therapeutic approaches. The diverse DNA methylation phenotypes resulting from CR can be measured through data, which in turn can provide some guidance for the implementation and evaluation of CR. However, this requires a comprehensive consideration of individual differences and other health indicators, and long-term follow-up studies are needed to verify its effects and safety[49]. Although lifestyle modifications can provide some temporary relief in delaying the progression of liver aging, their impact is constrained. Addressing age-related liver diseases necessitates targeting pivotal factors, including cellular senescence, the SASP, mitochondrial dysfunction, and other relevant mechanisms. An integrated therapeutic strategy is likely the most effective way to combat these diseases. It is hoped that more in-depth research on the detailed mechanisms of liver aging will be published in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C, Grade C
Novelty: Grade B, Grade B, Grade C, Grade C
Creativity or Innovation: Grade B, Grade B, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade C, Grade C
P-Reviewer: Horkaew P; Martinez-Molina C; Wang L S-Editor: Wei YF L-Editor: A P-Editor: Zhao S