Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.103921
Revised: February 22, 2025
Accepted: March 17, 2025
Published online: April 14, 2025
Processing time: 127 Days and 21 Hours
Current disinfection methods for gastrointestinal endoscopes consume a signifi
To achieve the objectives of efficiency, speed, and cost-effectiveness, this study utilized vaporized hydrogen peroxide (VHP) generated from sodium percarbonate granules to conduct an anhydrous disinfection test on gastrointestinal endoscopes.
The experimental device rapidly converts sodium percarbonate granules into VHP, and performs disinfection experiments on gastrointestinal endoscope mo
The device generates a certain concentration of VHP that can achieve high-level disinfection of endoscope models within 30 minutes. RH, exposure dosage, and organic burden significantly affect the disinfection efficacy of VHP, whereas the intraluminal FR does not significantly impact disinfection efficacy. All ten artificially contaminated disposable endoscopes achieved satisfactory disinfection results. Furthermore, when this device was used to treat various types of reusable endoscopes, the disinfection and sterilization effects were not significantly different from those of automatic endoscope disinfection machines (using peracetic acid disinfectant solution) (P > 0.05), and the economic cost of disinfectant required per endoscope was lower (1.5 China Yuan), with a shorter disinfection time (30 minutes).
The methods and results of this study provide a basis for further research on the use of VHP for the disinfection of gastrointestinal endoscopes, as well as for the development of anhydrous disinfection technology for gastroin
Core Tip: This study pioneers an anhydrous disinfection method for gastrointestinal endoscopes using vaporized hydrogen peroxide generated from sodium percarbonate granules. Demonstrating comparable efficacy to traditional liquid disinfectants, the method achieves high-level disinfection within 30 minutes while reducing water use, costs (1.5 China Yuan per cycle), and environmental impact. Key factors-humidity, exposure dosage, and organic burden-significantly influence efficacy, with no adverse effects from flow rate variations. This innovation aligns with green endoscopy goals, offering a sustainable, efficient alternative to resource-intensive reprocessing, and sets a foundation for future anhydrous sterilization technologies.
- Citation: Zhao C, Qi LH, Li LS, Wang YY, Liang T, Chai NL. Using vaporized hydrogen peroxide for anhydrous disinfection of gastrointestinal endoscopes. World J Gastroenterol 2025; 31(14): 103921
- URL: https://www.wjgnet.com/1007-9327/full/v31/i14/103921.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i14.103921
Gastrointestinal endoscopes are slender and complex medical devices with long lumens. Currently, with the rapid deve
To meet this demand, the aim of this study was to explore a new method of disinfecting endoscopes that completely eliminates liquid disinfectants and is based on solid and gaseous substances. Although ethylene oxide and formaldehyde are also gaseous disinfectants that can achieve sterilization levels, they are highly toxic, require aeration, and significantly extend the sterilization time. Vaporized hydrogen peroxide (VHP) has rapidly developed in the past decade as an efficient disinfectant that is expected to replace ethylene oxide gas for the disinfection and sterilization of endoscopes[6]. Hydrogen peroxide has demonstrated effective antimicrobial activity against a variety of organisms, including bacteria, bacterial spores, fungi, bacilli, and viruses, without producing carcinogenic or toxic residues[7]. Past studies have demon
Sodium percarbonate granules are an effective alternative to hydrogen peroxide solutions[10-12], offering greater stability and safety than concentrated hydrogen peroxide solutions. These granules degrade quickly, are environmentally friendly and are considered green disinfectants[13]. On the basis of the above principles, for this study we designed a device that rapidly converts sodium percarbonate granules into VHP for disinfection within the endoscope lumen. We utilized endoscope models, disposable endoscopes, and various types of reusable gastrointestinal endoscopes as research subjects to study the disinfection effects of VHP on endoscopes and to explore a new model for anhydrous disinfection of gastrointestinal endoscopes.
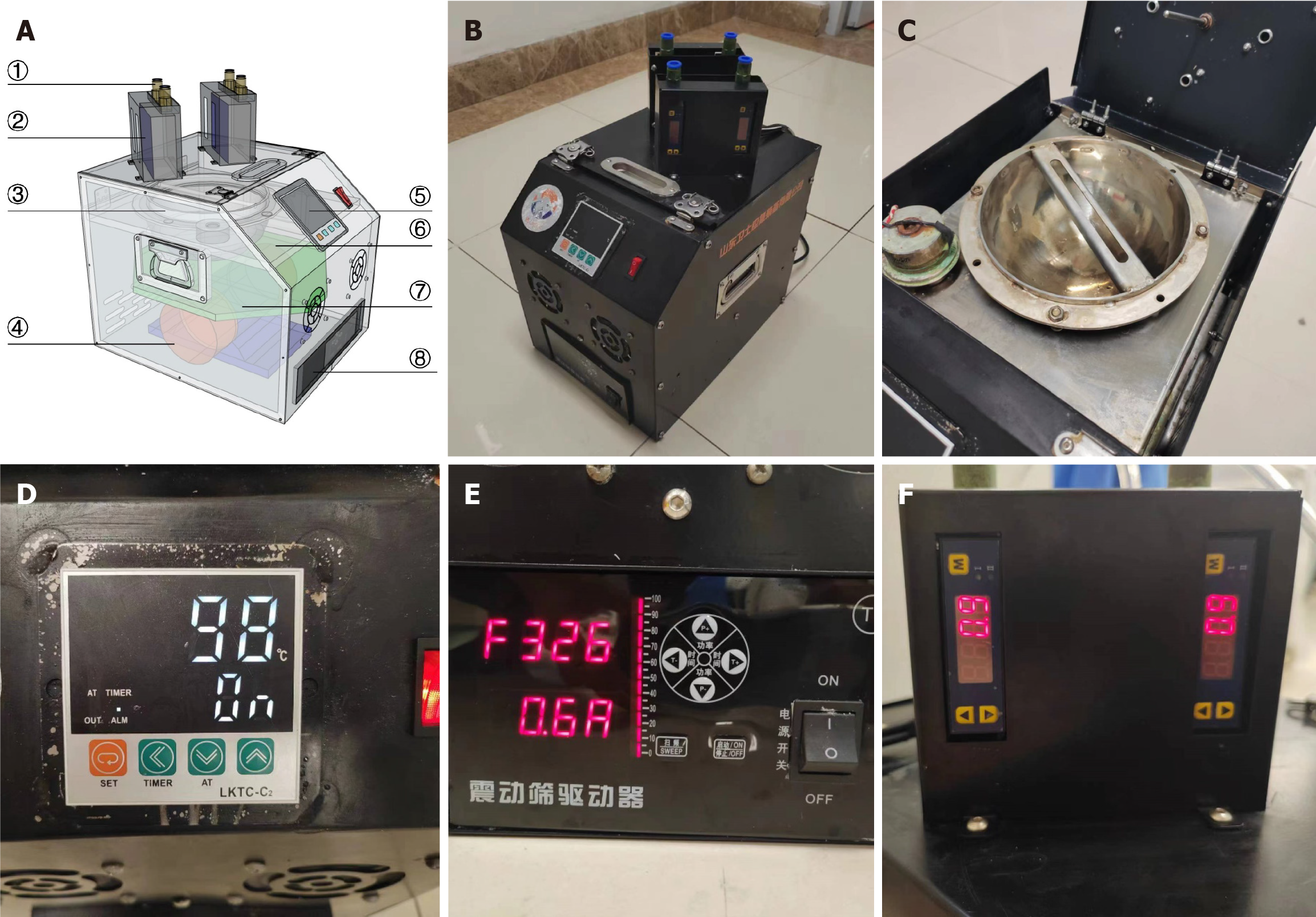
Figure 1 present the perspective and actual views (Figure 1A and B) of the experimental device designed and manufactured in this study, as well as detailed diagrams (Figure 1C-F) of some components. The device consists of four parts: The reaction vessel, heating control module, ultrasonic oscillation module, and flow control module. Sodium percarbonate granules were rapidly converted into VHP under the action of high-temperature heating and ultrasonic vibration. Then the gas was introduced into the endoscope lumen for disinfection through the gas nozzle at the top of the flow control module.
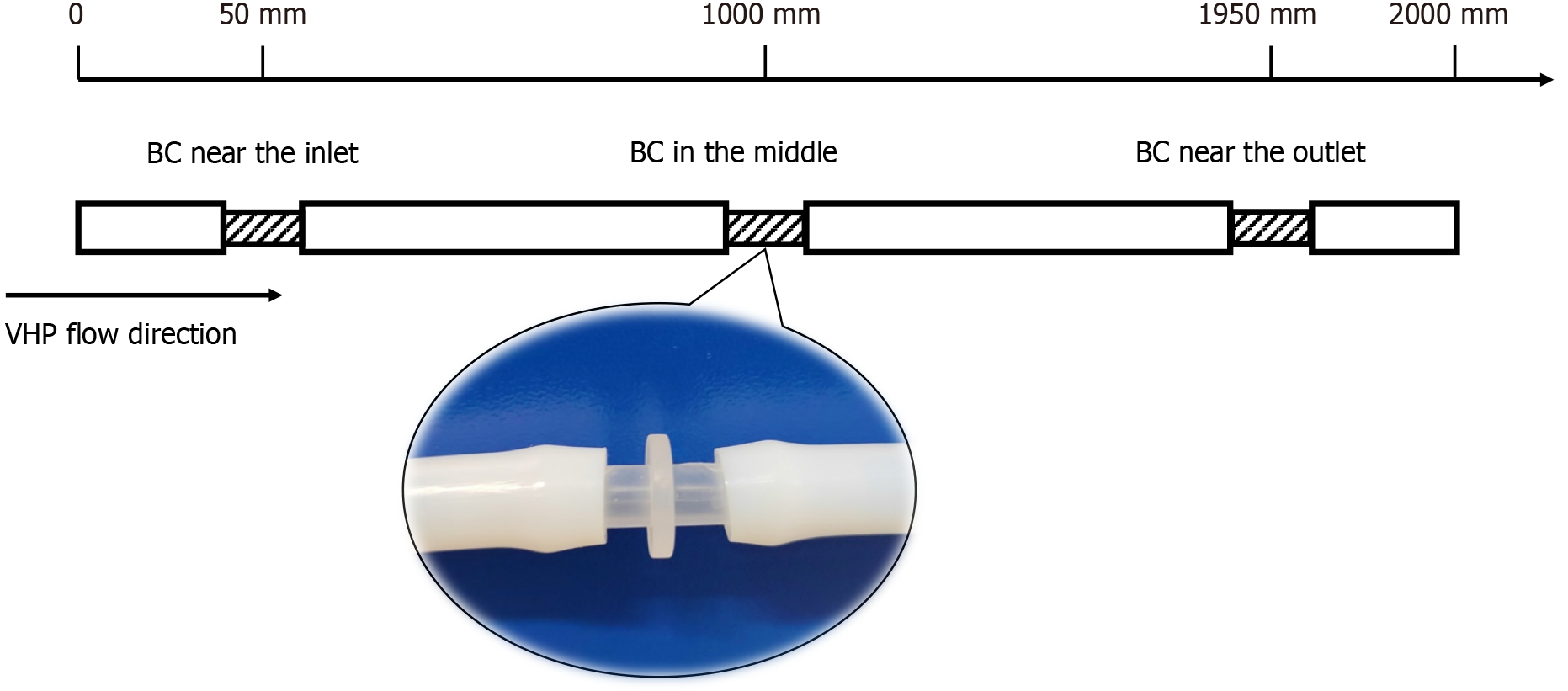
To adequately simulate the structure and material of a gastrointestinal endoscope, the endoscope model consisted of a polytetrafluoroethylene (PTFE) tube with an outer diameter of 8 mm, an inner diameter of 6 mm, and a total length of 2000 mm. The endoscope model was cut into four sections at the 50 mm, 1000 mm, and 1950 mm marks, with bacterial carriers inserted at the three cuts to simulate different contamination sites of the endoscope (Figure 2). The PTFE tubes were securely fitted with the bacterial carriers, and all the tubes were subjected to a degreasing process and autoclaved for sterilization before application. The design of the validation test referred to the Evaluation Method of Endoscopic Disinfection Effect (2020 Edition, China) and international organization for standardization (ISO) 22441: 2022 Sterilization of health care products - Low temperature vaporized hydrogen peroxide - Requirements for the development, validation and routine control of a sterilization process for medical devices.
This experiment refers to the Evaluation Method of Endoscopic Disinfection Effect (2020 Edition, China) and the contents of ISO 11138-6, Sterilization of health care products-Biological indicators before Bacillus subtilis (B. subtilis) spores (ATCC 9372) were used as a biological indicator. The diluent was tryptone physiological salt solution, and the culture medium was nutrient agar (9 cm, Beckman Biology). A pipette was used to drop 0.02 mL of spore suspension onto the inner wall of the PTFE tube carrier (outer diameter of 6 mm, inner diameter of 4 mm, and length of 30 mm), which had been defatted and sterilized by autoclaving, after which it was spread evenly. The recovery bacterial count for each carrier ranged from 1 × 106 to 5 × 106 colony-forming units (CFUs). All carriers with spores were placed on plates on a clean workstation for 30 minutes to dry before use. The prepared bacterial carriers were used within 24 hours of inoculation.
The device was placed inside a disinfection chamber with dimensions of 500 mm × 500 mm × 500 mm. The chamber was equipped with a VHP concentration sensor (HPP271, Vaisala, Finland), temperature and humidity sensors (HMD60Y, Vaisala, Finland), and a negative pressure ventilation pipe to allow for continuous monitoring of the working status of the disinfection device. During disinfection, the sodium percarbonate granules were first placed into the reaction vessel. All parameters of the device were set, the endoscope model or the various channels of the endoscopes were connected, and the heating and ultrasonic vibration modules were turned on. Approximately 5 minutes later, when the VHP concentration in the chamber reached its peak, the timing began. After disinfection was complete, the residues were removed, and disinfection was subsequently performed.
Laboratory experiments: The contaminated carriers were connected to the gas generation device nozzle, the disinfection device was activated, the timing was started after 5 minutes, and disinfection was applied for 10 minutes, 20 minutes, and 30 minutes, respectively. After disinfection, the carriers were sampled and incubated in a 36 ± 1 °C incubator for 72 hours. The results were observed, the viable bacteria were counted, and those bacteria were considered the experimental group. For each exposure time, three carriers were selected for parallel experiments, and each carrier was inoculated into two Petri dishes for cultivation. Additionally, the same contaminated carriers were connected to the gas generation device nozzle, the device was continuously turned off, and after 30 minutes, the carriers were removed for cultivation. Again, the same steps were followed, the viable bacteria were counted, and these bacteria were considered the positive control group. Additionally, two uncontaminated carriers were subjected to similar steps as those in the negative control group. The experiment was repeated three times, the viable bacterial counts (CFU/carrier) for each group were calculated, and the log reduction value was calculated via the formula KL = N0 - NX, where N0 and NX represent the CFUs recovered from the positive control and disinfected carriers, respectively. The results were considered valid if the positive control group showed bacterial growth, the negative control group showed no bacterial growth, and the experimental group had a log reduction value of ≥ 5.00.
Endoscope model disinfection tests: The endoscope models with contaminated carriers were connected to the nozzle of the device and disinfection began. After disinfection was complete, the carriers were removed from various parts, samples were taken, and the samples were placed in a 36 ± 1 °C incubator for 72 hours for cultivation. The results were observed, and viable bacteria in the experimental group were counted. Additionally, the same endoscope model was taken and connected to the device's nozzle with the disinfection device continuously turned off. After 30 minutes, the carriers were removed, and the same steps were followed for cultivation as mentioned above, with the positive control group considered. Additionally, physiological saline was inoculated onto Petri dishes for 72 hours for cultivation, the results were observed, and viable bacteria were counted as the negative control group. The experiment was repeated three times, and the log reduction values for each group were calculated. The results were considered valid if the positive control group showed bacterial growth, the negative control group showed no bacterial growth, and the experimental group had a log reduction value of ≥ 3.00. Previous research results have indicated that relative humidity (RH), the intraluminal flow rate (FR), the exposure dosage, and the organic burden may be the main factors affecting the effectiveness of gas disinfection[14,15]; therefore, these factors are used as variables to further explore the disinfection parameters of VHPs.
Disposable endoscope disinfection tests: Ten disposable endoscopes (EndoFresh) were selected for disinfection testing. After rigorous cleaning and disinfection, the samples were kept ready for use. A pipette was used to inject a spore suspension equivalent in volume to that of the simulated endoscope into the biopsy channel entrance and the suction channel of the endoscope. The height of the endoscope channels was adjusted to evenly distribute the bacterial mixture within the lumen. The surface of the endoscope was wiped with gauze soaked in the bacterial mixture and placed on a clean workstation for 1 hour before use. Before the disinfection test, prewashing, cleaning, and final rinsing steps were performed for each endoscope. Swab samples were taken from the biopsy port, surface, suction channel, and lumen of each endoscope; these swab samples were then used for culture. After disinfection, samples were taken again from each part, and the total number of colonies ≤ 20 CFU per item was considered valid.
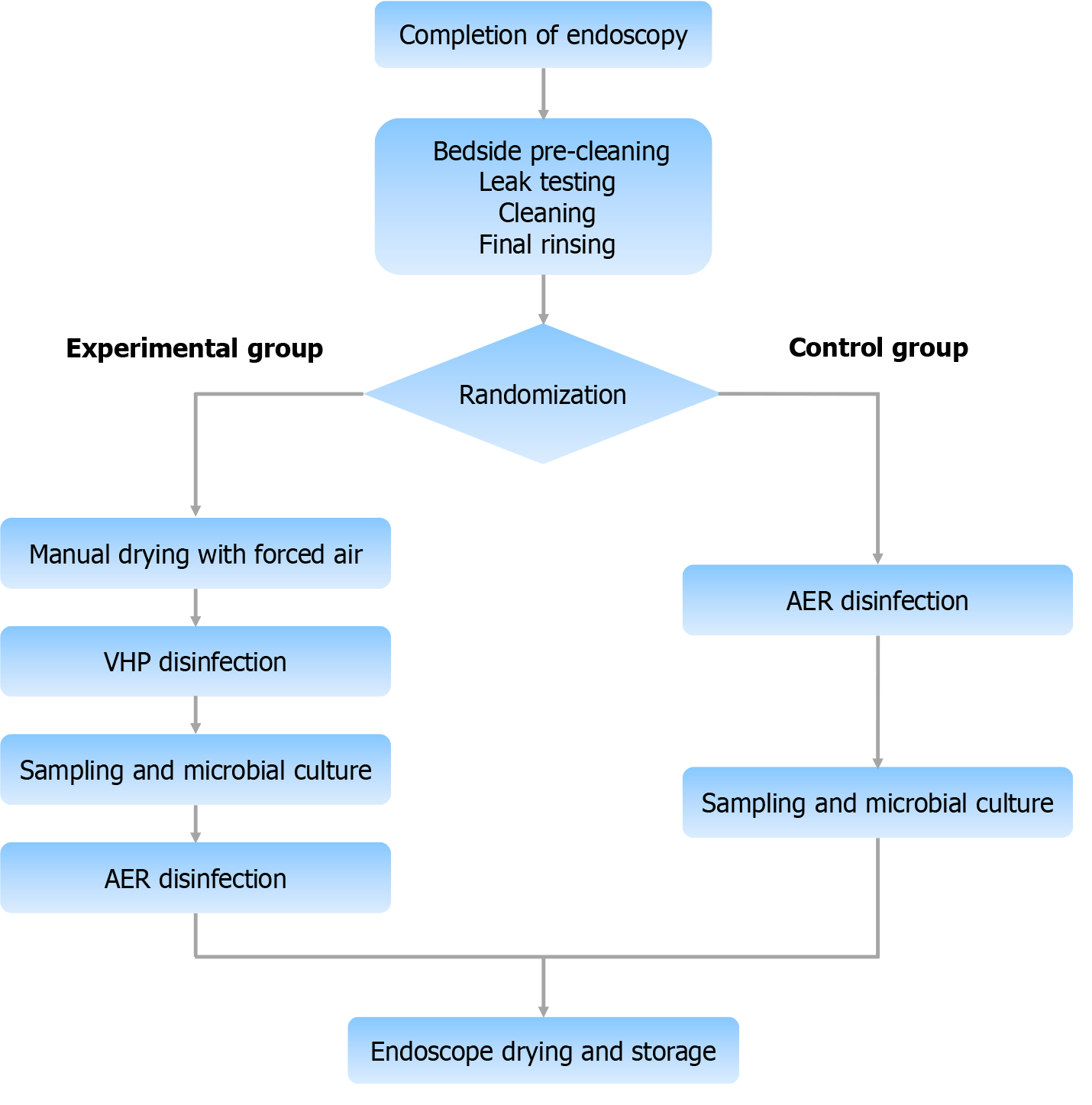
Reusable endoscope disinfection tests: A total of 120 reusable gastrointestinal endoscopes (including 40 gastroscopes, 40 colonoscopes, and 40 duodenoscopes) were selected, and each type of endoscope was randomly assigned into groups. One group was the experimental group, which was disinfected via a VHP disinfection device, and samples were collected for testing after disinfection. After sampling, the experimental group was disinfected again via an automated endoscope reprocessor (AER) to ensure full compliance with the disinfection process. Another group was the control group, which was disinfected with an AER (peracetic acid solution), and samples were collected for testing after disinfection. The experimental group underwent a drying step before disinfection to reduce the impact of residual droplets absorbing VHP by the disinfection effect (Figure 3). Disinfection was considered valid if the total colony count was ≤ 20 CFU per item and no pathogenic bacteria were present; sterilization was achieved if no colonies were detected throughout the endoscope. The disinfection and sterilization effectiveness of the two groups were compared.
SPSS statistics software (version 26.0) was used for analysis of variance and χ2 tests of the experimental data. P values of less than 0.05 were considered statistically significant.
Seventy-five g of sodium percarbonate granules were added to the device, and the contaminated carriers were disinfected for 10 minutes, 20 minutes, or 30 minutes. The results, as shown in Table 1, indicate that when the disinfection time is 30 minutes, the log reductions for all carriers are > 5.00. The positive control group recovered an average colony count of 4.5-4.8 × 106 CFU, and no colonies grew in the petri dishes of the negative control group, demonstrating that the VHP produced by 75 g of sodium percarbonate granules can achieve a high-level disinfection (HLD) effect on the B. subtilis spores on the carriers within 30 minutes.
| Disinfection time (minutes) | Total | Unqualified | Qualified | Average CFUs of the positive control group (CFU/bacterial carrier) |
| 10 | 8 | 8 | 0 | 4.8 × 106 |
| 20 | 8 | 2 | 6 | 4.5 × 106 |
| 30 | 8 | 0 | 8 | 4.7 × 106 |
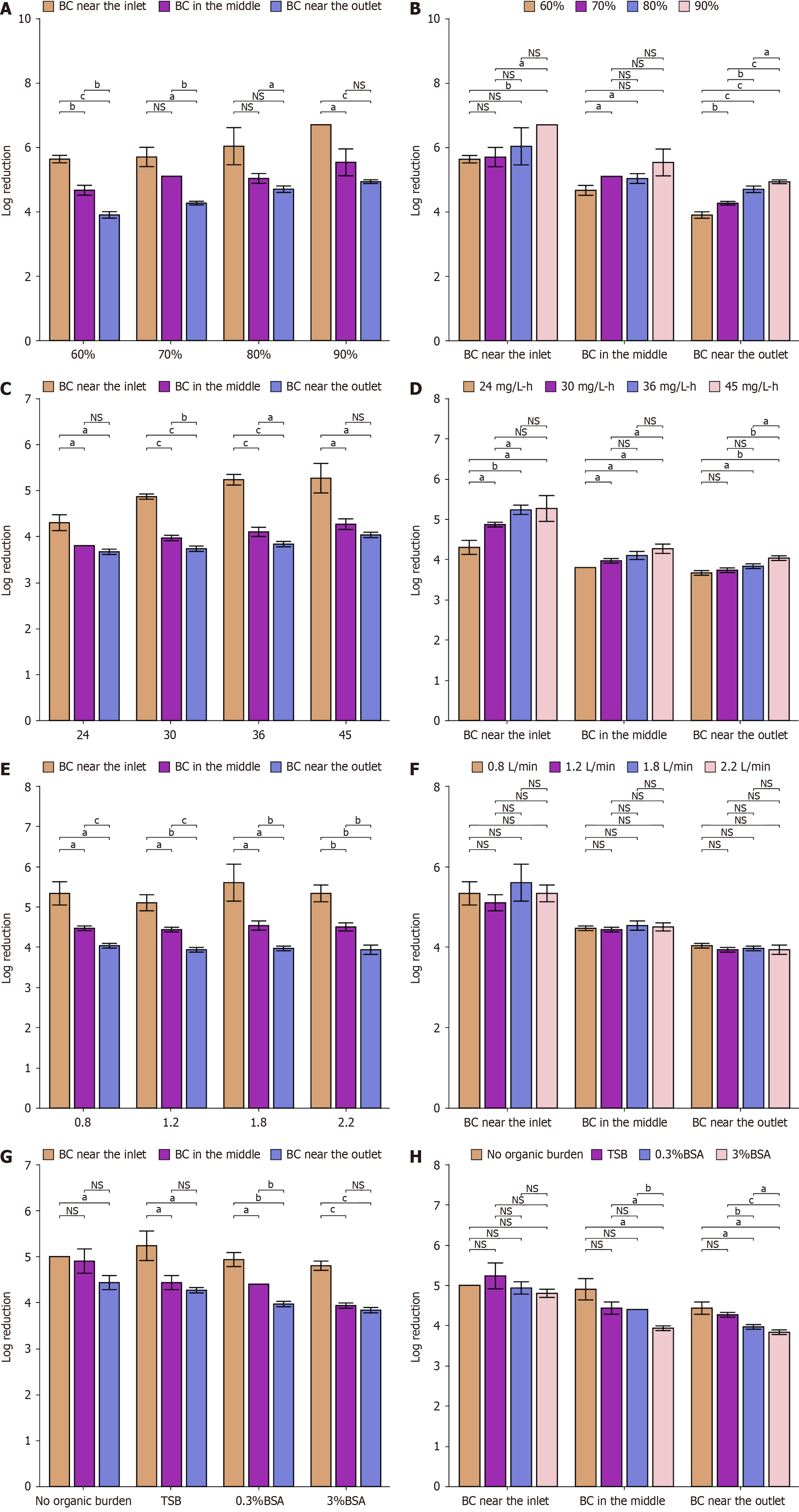
Disinfection efficiencies of VHP with various RHs: Figure 4A and B show the log reduction of spores on bacterial carriers treated with 90 mg/L VHP for 30 minutes with an FR of 2.2 L/minutes and various RHs (60%-90%). In general, the disinfection efficiency of VHP for bacterial carriers at the same position increased as the RH increased, which was more pronounced at the outlet. The log reductions of the contaminated carriers at different positions were all > 3.00.
Disinfection efficiencies of VHP at various exposure dosages: As shown in Table 2, different exposure dosages were administered. Figure 4C and D show the disinfection efficiency of VHP in the inactivation of spores on bacterial carriers with various exposure dosages (24-45 mg/L/hour), 80% RH and 2.2 L/minutes FR. The results indicate that the disin
| Concentration of VHP (mg/L) | Disinfection time (minute) | Exposure dosage (mg/L/hour) |
| 72 | 20 | 24 |
| 90 | 20 | 30 |
| 72 | 30 | 36 |
| 90 | 30 | 45 |
Disinfection efficiencies of VHP with various intraluminal FRs: The log reductions of spores on bacterial carriers following the 30 minutes treatment with 90 mg/L VHP, 80% RH, and various FRs (0.8-2.2 L/minutes) are shown in Figure 4E and F. In general, the intraluminal FR did not significantly affect the disinfection efficacy of VHP (P > 0.05). The log reductions of the contaminated carriers at different positions were all > 3.00.
Disinfection efficiencies of VHP with various organic burden: Figure 4G and H show the log reductions of spores on bacterial carriers treated with 90 mg/L VHP for 30 minutes with 80% RH, 2.2 L/minutes FR and various organic burdens. The results show that the organic burden has a significant effect on the disinfection efficacy of VHP, which is reflected mainly in the bacterial carrier at the outlet. Tryptone soy broth (TSB) has little effect on gas disinfection, but higher concentrations of bovine serum albumin (BSA) can reduce the disinfection effect of VHP. The log reductions of the con
Table 3 presents the results of disinfecting disposable endoscopes with VHP. After the predisinfection cleaning steps, a small amount of microbial load still existed in various parts of the endoscope. No viable bacteria were detected in any parts of the disposable endoscopes following the 30 minutes treatment with 90 mg/L VHP, 90% RH, and 2.2 L/minutes FR.
| No. | Number of colonies (CFU/item) | |||||||
| Biopsy channel | Biopsy valve | Endoscope surface | Suction channel | |||||
| BD | AD | BD | AD | BD | AD | BD | AD | |
| 1 | 10 | 0 | 0 | 0 | 10 | 0 | 19 | 0 |
| 2 | 40 | 0 | 0 | 0 | 0 | 0 | 24 | 0 |
| 3 | 140 | 0 | 38 | 0 | 7 | 0 | 6 | 0 |
| 4 | 30 | 0 | 12 | 0 | 11 | 0 | 0 | 0 |
| 5 | 10 | 0 | 12 | 0 | 0 | 0 | 0 | 0 |
| 6 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 100 | 0 | 18 | 0 | 42 | 0 | 102 | 0 |
| 9 | 20 | 0 | 25 | 0 | 0 | 0 | 58 | 0 |
| 10 | 80 | 0 | 12 | 0 | 18 | 0 | 7 | 0 |
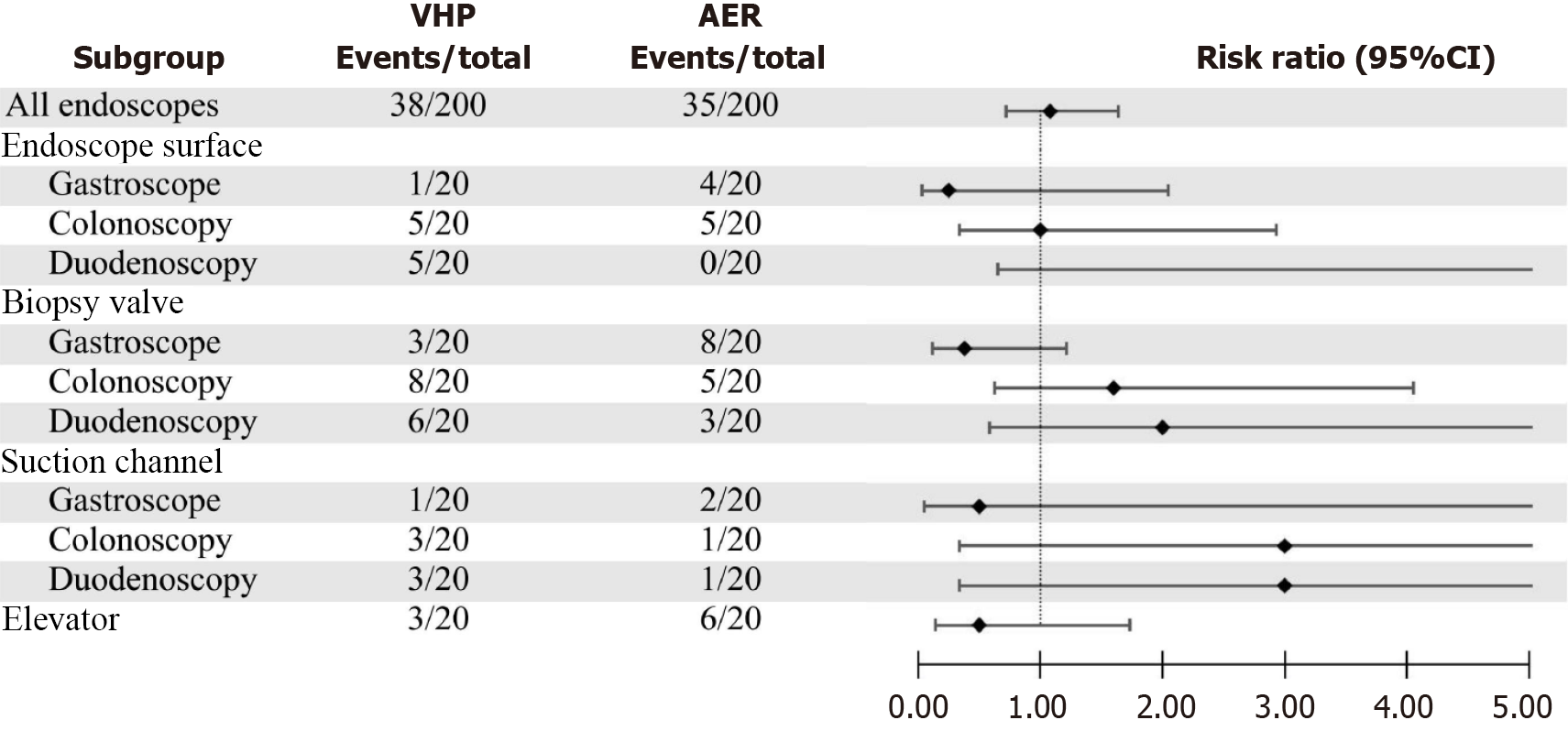
Figure 5 illustrates the application scenario of the prototype for disinfecting reusable endoscopes. Tables 4 and 5 compare the HLD and sterilization effects, respectively, of the two methods on gastrointestinal endoscopes. The results indicate that the prototype can essentially achieve the same level of endoscope treatment capability as an AER, with no significant difference in the effects of HLD or sterilization (P > 0.05). Figure 6 shows the comparison of the presence of colonies at different locations of the gastrointestinal endoscopes after treatment by the two reprocessing methods, which revealed no significant difference between the two methods. Moreover, 11 gastrointestinal endoscopes used by patients with positive serology for hepatitis B surface antigen (HBSAg), anti-hepatitis C virus (HCV) antibodies, and TP-Ab were selected for the experiment. After disinfection with the prototype, the abovementioned endoscopes were negative for HBsAg, anti-HCV antibodies, and TP-Ab.
| Group | Gastroscope (n = 40) | Colonoscopy (n = 40) | Duodenoscopy (n = 40) | All endoscopes (n = 120) |
| Experimental group | 19 (95) | 18 (90) | 18 (90) | 55 (91.6) |
| Control group | 20 (100) | 20 (100) | 20 (100) | 60 (100) |
| χ2 | 0.000 | 0.526 | 0.526 | 3.339 |
| P value | 1.000 | 0.468 | 0.468 | 0.068 |
| Group | Gastroscope (n = 40) | Colonoscopy (n = 40) | Duodenoscopy (n = 40) | All endoscopes (n = 120) |
| Experimental group | 15 (75) | 10 (50) | 10 (50) | 35 (58.3) |
| Control group | 11 (55) | 13 (65) | 13 (65) | 37 (61.7) |
| χ2 | 1.758 | 0.921 | 0.921 | 0.198 |
| P value | 0.185 | 0.337 | 0.337 | 0.656 |
Despite continuous innovations in the types and methods of disinfectants, there are still numerous reports of endoscope-related infection incidents globally each year[16,17]. Current disinfection methods consume a considerable amount of water resources and produce a large amount of waste. VHP is a highly efficient, green disinfectant that has been utilized in various fields. However, hydrogen peroxide solutions are very susceptible to decomposition during long-term storage and transportation, light exposure, stirring, and heating, which all accelerate the rate of decomposition[18,19]. Sodium percarbonate has been used as a solid alternative to hydrogen peroxide solutions, offering advantages such as non
Compared with an AER device, the prototype device can treat any type of gastrointestinal endoscope in just 30 minutes via VHP produced from 150 g of sodium percarbonate granules, with disinfection and sterilization effects showing no significant difference. The disinfection time is shorter than that of an AER or peracetic acid solution (37 minutes for gastroscopes and colonoscopes and 68 minutes for duodenoscopes). The cost of the disinfectant (sodium percarbonate granules) is only 1.5 China Yuan (CNY) per endoscope, which is significantly lower than the 28.7 CNY per endoscope for peracetic acid disinfectant solution, and the solid granules require much less storage space than do the liquid disin
Notably, this anhydrous disinfection method requires two drying processes, which may increase the workload of the disinfection staff.
Biological indicators are microorganisms that possess greater resistance than the microorganisms killed on medical devices[6]. Among the different types of microorganisms, spores of gram-positive bacteria are the most resistant to chemical disinfectants. Therefore, if a disinfectant is able to disinfect spores of gram-positive bacteria to a certain degree, it can theoretically inactivate all other pathogens as well[21]. B. subtilis spores have been widely used as biological indicators for the disinfection of various gaseous disinfectants[22]. Therefore, it was selected as the sole biological indicator bacterium in this study.
This study revealed that the exposure dose, RH, and organic burden can affect the killing effect of VHP on spores. The higher the exposure dose is, the better the disinfection effect of VHP. On the basis of this result, the concentration of VHP can be increased to achieve faster disinfection. With respect to the impact of RH on the disinfection effect of VHP, Beatriz from Germany conducted relevant experiments[23], whose study revealed that under relatively low VHP concentrations (≤ 400 mg/L), relatively high RH would increase the disinfection level. Additionally, higher humidity conditions cause water molecules to expand the volume of spores, and the increased surface area of the spores increases the susceptibility of the spore wall to breakthrough by VHP[24,25]. Similar results were obtained in this study, where the killing effect on spores increased with increasing RH at a VHP concentration of 90 mg/L. Therefore, increasing the environmental RH may be beneficial for improving disinfection efficacy, reducing the amount of disinfectant, lowering disinfection costs, and minimizing the potential damage of VHP to endoscopes.
The presence of organic matter on the surface of endoscopes can hinder contact between disinfectants and microorganisms, reduce the killing effect, and facilitate the formation of biofilms, greatly increasing the risk of endoscope-related infections[26]. In this study, 3% BSA significantly reduced the disinfection effect of VHP compared with 0.3% BSA, whereas TSB did not have a significant interfering effect. This fully demonstrates the importance of cleaning to remove a large organic load before disinfection[27].
Furthermore, the results of this study show that the variation in FRs in the lumen did not significantly affect the killing effect of VHP on spores. Some studies have indicated that VHP has a greater ability to penetrate three-dimensional structures composed of proteins, lipids, and polysaccharides than hydrogen peroxide, and VHP is more capable of damaging the spore wall and subsequently the DNA within spores[7,28,29]. The variation in the FR within the range of 0.8-2.2 L/minutes may not be sufficient to affect the destruction of spores by VHP, and further research is needed to determine the specific threshold that would make a difference.
Although this study confirms the efficacy of hydrogen peroxide gas in disinfecting the lumen of digestive endoscopes, its penetration is still the focus of international controversy. The presence of hydrogen peroxide gas in the slender lumen may be due to high diffusion resistance and uneven gas distribution, resulting in insufficient local concentration, thus affecting disinfection efficacy. This limitation is confirmed by the significantly lower number of log spores killed at the exit of the simulated endoscope model than at the entry point in this experiment. The following improvement directions can be considered: One is to optimize the aerodynamic mode, using pulsed gas flow instead of continuous gas flow, enhancing the penetration depth of gas in the lumen through pressure fluctuations, and integrating multidirectional nozzles in the device to inject gas bidirectionally from the biopsy port and suction port of the endoscope to reduce the "dead space" effect; the other is to install a hydrogen peroxide concentration sensor at the end of the lumen, dynamically adjusting the gas flow and exposure time to ensure that the minimum effective concentration is up to the standard during the whole process; and the third is collaborative technology integration, which uses the cavitation effect of low-frequency ultrasound (20-40 kHz) to destroy the biofilm structure, improve the contact efficiency between gas and microorganisms, and introduce plasma activation technology to stimulate hydrogen peroxide gas to generate reactive oxygen species, enhancing the killing ability of deep pollution.
Although this test verified the effects of RH, exposure dosage, and organic burden on the disinfection of VHP, the optimal operating parameters of the device were not further investigated to explore the disinfection potential of VHP. The test device could disinfect only one endoscope at a time, and its effect on the simultaneous disinfection of multiple endoscopes was not investigated. The potential damage of VHP to endoscopic equipment, which is closely related to the disinfection concentration and time, was not further investigated.
The experimental results indicate that treating gastrointestinal endoscopes with VHP generated from sodium percarbonate granules can essentially achieve the disinfection effect of an AER and peracetic acid mixture, significantly reducing the cost of disinfectants. Moreover, the entire disinfection process greatly reduces the use of water. By adjusting parameters such as gas concentration and RH, the disinfection time can be greatly shortened; this provides valuable ideas for achieving rapid and anhydrous disinfection, as well as realizing the concept of green endoscopy.
We sincerely thank the peer reviewers for their comprehensive and constructive suggestions on this manuscript.
| 1. | Day LW, Muthusamy VR, Collins J, Kushnir VM, Sawhney MS, Thosani NC, Wani S. Multisociety guideline on reprocessing flexible GI endoscopes and accessories. Gastrointest Endosc. 2021;93:11-33.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Vaccari M, Tudor T, Perteghella A. Costs associated with the management of waste from healthcare facilities: An analysis at national and site level. Waste Manag Res. 2018;36:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 3. | Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 475] [Article Influence: 158.3] [Reference Citation Analysis (1)] |
| 4. | Le NNT, Hernandez LV, Vakil N, Guda N, Patnode C, Jolliet O. Environmental and health outcomes of single-use versus reusable duodenoscopes. Gastrointest Endosc. 2022;96:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Park SB, Cha JM. Gastrointestinal endoscopy's carbon footprint. Clin Endosc. 2023;56:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 6. | McEvoy B, Rowan NJ. Terminal sterilization of medical devices using vaporized hydrogen peroxide: a review of current methods and emerging opportunities. J Appl Microbiol. 2019;127:1403-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother. 2012;67:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 298] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 8. | Finnegan M, Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY. Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. J Antimicrob Chemother. 2010;65:2108-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Omidbakhsh N, Manohar S, Vu R, Nowruzi K. Flexible gastrointestinal endoscope processing challenges, current issues and future perspectives. J Hosp Infect. 2021;110:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Zhang YH, Xue CM, Guo CH. Application Sodium Percarbonate to Oxidative Degradation Trichloroethylene Contamination in Groundwater. Procedia Environ Sci. 2011;10:1668-1673. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Viisimaa M, Goi A. Use of hydrogen peroxide and percarbonate to treat chlorinated aromatic hydrocarbon-contaminated soil. J Environ Eng Landsc. 2014;22:30-39. [DOI] [Full Text] |
| 12. | Goi A, Viisimaa M, Trapido M, Munter R. Polychlorinated biphenyls-containing electrical insulating oil contaminated soil treatment with calcium and magnesium peroxides. Chemosphere. 2011;82:1196-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Więckol-Ryk A, Thomas M, Białecka B. Solid Peroxy Compounds as Additives to Organic Waste for Reclamation of Post-Industrial Contaminated Soils. Materials (Basel). 2021;14:6979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Park S, Kang D. Antimicrobial effect of chlorine dioxide gas against foodborne pathogens under differing conditions of relative humidity. LWT - Food Sci Technol. 2015;60:186-191. [RCA] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Yi Y, Hao LM, Ma SR, Wu JH, Wang T, Lin S, Zhang ZX, Qi JC. A pilot study on using chlorine dioxide gas for disinfection of gastrointestinal endoscopes. J Zhejiang Univ Sci B. 2016;17:526-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Aumeran C, Poincloux L, Souweine B, Robin F, Laurichesse H, Baud O, Bommelaer G, Traoré O. Multidrug-resistant Klebsiella pneumoniae outbreak after endoscopic retrograde cholangiopancreatography. Endoscopy. 2010;42:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Khoury T, Gincul R, Mohammedi I, Sbeit W, Napoléon B. Antibioprophylaxis in endoscopic ultrasound guided fine needle aspiration in pancreatic cysts: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 18. | Li Y, Zhu Y, Wang D, Yang G, Pan L, Wang Q, Ni BJ, Li H, Yuan X, Jiang L, Tang W. Fe(II) catalyzing sodium percarbonate facilitates the dewaterability of waste activated sludge: Performance, mechanism, and implication. Water Res. 2020;174:115626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 19. | Ling X, Deng J, Ye C, Cai A, Ruan S, Chen M, Li X. Fe(II)-activated sodium percarbonate for improving sludge dewaterability: Experimental and theoretical investigation combined with the evaluation of subsequent utilization. Sci Total Environ. 2021;799:149382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Gao J, Duan X, O'Shea K, Dionysiou DD. Degradation and transformation of bisphenol A in UV/Sodium percarbonate: Dual role of carbonate radical anion. Water Res. 2020;171:115394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Luftman HS, Regits MA. B. Atrophaeus and G. Stearothermophilus Biological Indicators for Chlorine Dioxide Gas Decontamination. Appl Biosaf. 2008;13:143-157. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Sella SR, Vandenberghe LP, Soccol CR. Bacillus atrophaeus: main characteristics and biotechnological applications - a review. Crit Rev Biotechnol. 2015;35:533-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Unger-Bimczok B, Kottke V, Hertel C, Rauschnabel J. The Influence of Humidity, Hydrogen Peroxide Concentration, and Condensation on the Inactivation of Geobacillus stearothermophilus Spores with Hydrogen Peroxide Vapor. J Pharm Innov. 2008;3:123-133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Westphal AJ, Price PB, Leighton TJ, Wheeler KE. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc Natl Acad Sci U S A. 2003;100:3461-3466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Young SB, Setlow P. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J Appl Microbiol. 2003;95:54-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Weber DJ, Rutala WA, Anderson DJ, Sickbert-Bennett EE. Biofilms on medical instruments and surfaces: Do they interfere with instrument reprocessing and surface disinfection. Am J Infect Control. 2023;51:A114-A119. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Rutala WA, Gergen MF, Weber DJ. Does blood on "dirty" instruments interfere with the effectiveness of sterilization technologies? Infect Control Hosp Epidemiol. 2022;43:1262-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Mcdonnell G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In: Patai's Chemistry of Functional Groups. NJ: John Wiley & Sons, Ltd., 2014. [DOI] [Full Text] |
| 29. | McEvoy B, Eveland R. Vaporized Hydrogen Peroxide: A Well-Known Technology with a New Application. Biomed Instrum Technol. 2020;54:74-79. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |