Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.103901
Revised: March 3, 2025
Accepted: March 25, 2025
Published online: April 14, 2025
Processing time: 128 Days and 14.2 Hours
Colorectal cancer (CRC), the third most prevalent cancer globally, exhibits a notable association with venous thromboembolism (VTE), significantly impacting patient morbidity and mortality. We delve into the complex pathogenesis of cancer-associated thrombosis (CAT) in CRC, highlighting the interplay of clinical risk factors and tumor-specific mechanisms. Our comprehensive review synthe
Core Tip: This comprehensive review examines the complex interplay between genetic and clinical factors in colorectal cancer (CRC)-associated thrombosis. It highlights how tumor-specific mechanisms, such as tissue factor and cancer procoagulant, actively contribute to the coagulation cascade, alongside clinical risk factors like age, gender, and obesity. By integrating both tumor biology and patient-specific characteristics, this study provides crucial insights into the prothrombotic tendencies of CRC, offering valuable perspectives for personalized therapies and improving patient management in CRC-associated venous thromboembolism.
- Citation: Xu DG, Tan J. Interplay of genetic and clinical factors in cancer-associated thrombosis: Deciphering the prothrombotic landscape of colorectal cancer. World J Gastroenterol 2025; 31(14): 103901
- URL: https://www.wjgnet.com/1007-9327/full/v31/i14/103901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i14.103901
As of 2020, colorectal cancer (CRC) ranks as the third most common cancer globally and produces the second-highest mortality rate among cancers[1]. The intriguing association between idiopathic thromboses and concealed malignancies was first proposed in 1865 by Armand Trousseau, who identified superficial thrombophlebitis as a potential early marker of hidden visceral cancers[2]. Notably, CRC exemplifies this connection, with reports citing CRC as presenting one of the highest incidences of thrombotic events among various prevalent cancers[3]. Moreover, hypercoagulable states in patients with CRC have been associated with both clinical progression and prognosis.
The intricate association between cancer and venous thromboembolism (VTE) is widely acknowledged, although its etiology remains a subject of ongoing research. Notably, about 20% of patients with VTE are concurrently diagnosed with cancer, and patients with cancer exhibit a substantially heightened risk of VTE, ranging from 14% to 25%[4-6]. This risk is most pronounced during the initial three months following a cancer diagnosis[7,8]. Moreover, cancer-associated thrombosis (CAT) significantly contributes to both morbidity and mortality, with VTE ranking as the second leading cause of death in patients with cancer, following the cancer itself[9,10]. Patients with metastatic cancers, in particular, have a higher thrombosis risk than those with non-metastatic forms. Even in the absence of overt thrombosis, most patients with cancer exhibit hypercoagulability, detectable through laboratory testing[4]. Moreover, cancer treatments, including surgery and chemotherapy, are known to elevate the VTE risk[11,12].
The pathogenesis of CAT in CRC is complex and multifaceted. Clinical factors encompassing general risks such as advanced age, being a woman, obesity, prior VTE, infection, and surgery; along with tumor-specific factors like tumor location, advanced stage, and metastatic disease, contribute to the thrombotic risk in patients with CRC. Moreover, targeted vascular therapy is a notable risk factor. CAT pathogenesis involves diverse mechanisms. For instance, tumor cells can activate the host’s hemostatic system in multiple ways, often driven by oncogenes implicated in neoplastic transformation[13]. These tumor tissues express an array of procoagulant proteins, including tissue factor (TF), cancer procoagulant (CP), and various coagulation factors. They also shed procoagulant-associated extracellular vesicles (EVs) and can induce procoagulant properties in host cells. This induction occurs either through direct cell-cell adhesions or by releasing inflammatory cytokines and proangiogenic factors.
In this article, we examine the progress in understanding the thrombogenicity of CRC, with a special emphasis on the mechanisms underlying tumor-specific activation of procoagulant properties.
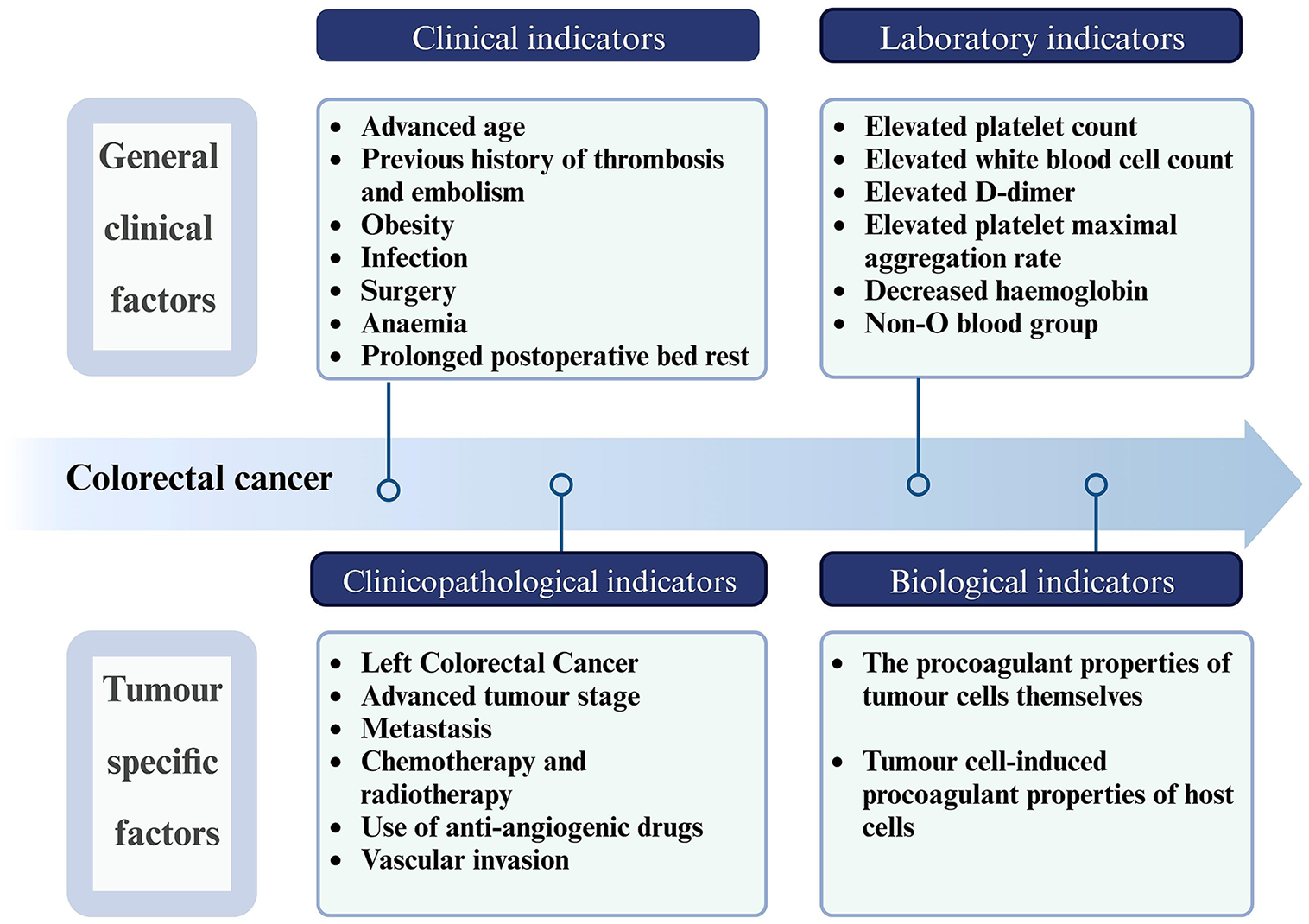
Clinical risk factors for thrombosis in CRC encompass both general clinical and tumor-specific factors. These factors are prevalent in all sorts of patients with and without cancer. However, the presence of tumor-specific clinical risk indicators and biological markers in patients with malignant tumors lends a unique aspect to the pathogenesis of thromboses associated with CRC. The interplay of these factors results in a shift of the hemostatic balance towards a hypercoagulable state that differentiates the thrombotic process in CRC from other conditions (Figure 1).
General clinical factors are pertinent to both oncological and non-oncological patients. These factors encompass a range of conditions, including age-related risks (particularly in elderly patients), a history of thrombosis and embolism, obesity, infection, surgical history, anemia, and prolonged immobilization post-surgery. Notably, sex is an independent risk factor for CAT in patients with CRC. A retrospective study by a Japanese scholar suggested that being women is an inde
Tumor location is a predominant tumor-specific factor. CRCs on the left side (encompassing rectal, sigmoid, and descending colon cancers) demonstrate a higher propensity for thrombosis than right-sided cancers (including ascending and transverse colon cancers). This tendency is attributed to higher blood viscosity, slower blood flow, and increased intestinal inflammation associated with left-sided CRC tumors, which may promote thrombosis[26,27]. Epidemiological data indicate that certain malignancies, like brain, hematological, pancreatic, gastric, and ovarian cancers, exhibit higher VTE risks than others[28,29]. Cancer stage is another crucial risk determinant, with advanced metastatic diseases posing a greater VTE risk than localized tumors[29]. In patients with advanced CRC, increased tumor burden and systemic inflammatory response promote the expression of procoagulant molecules[30]. Advanced tumors can obstruct or compress blood vessels, leading to stagnant blood flow, an important component of the Virchow’s triad that leads to thrombosis[31]. In addition, the presence of central venous catheters for long-term chemotherapy or parenteral nutrition in patients with advanced cancer can disrupt normal blood flow and lead to thrombosis[32]. The period immediately following a cancer diagnosis is characterized by a high VTE risk[7] probably due to the procedures and treatments common during this time: Diagnostic procedures, including colonoscopic biopsies, can lead to endothelial damage, generating a prothrombotic state and increasing the VTE risk. Many patients undergo surgical procedures such as colonoscopy or tumor resection after being diagnosed with CRC. In addition, treatments such as preoperative neoadjuvant chemotherapy may be initiated soon after diagnosis, further increasing the VTE risk.
The pathological tumor type may also influence the CAT risks. Mucinous CRC secretes excessive mucin as one of its distinguishing features. Studies have shown that TF-free oncolytic mucin preparations induce the formation of platelet-rich microthrombi in mice in a P-selectin-dependent and thrombin-independent manner[33,34]. Two other studies have also suggested that circulating Mucin1 (MUC1) attachment to EVs and other soluble mucins may also have a key role in thrombus formation[35,36]. However, the most recent study by Kawano et al[37] showed similar MUC1 levels in patients with cancer with or without VTE, and the data did not reveal any evidence of MUC1 being associated with VTE in patients with cancer. The conflicting evidence may be due to differences in mouse thrombosis models and the fact that Kawano et al[37] used a nested case-control study with a small number of patients, which led to results that are not generalizable. In the future, larger prospective cohort studies are needed to compare the role of mucins in thrombosis in tumors such as CRC. Therefore, the procoagulant mechanism of mucins in CRC needs further investigation.
Antitumor interventions, including chemotherapy, radiotherapy, antiangiogenic medication, and cancer surgery, can exert prothrombotic effects[38]. Chemotherapeutic agents and tumor derivatives compromise the vascular endothelial integrity, diminishing its antithrombotic properties. They also induce overexpression of TF and amplify cell membrane phosphatidylserine (PS) exposure[39]. Specifically, for chemotherapeutic agents, the oxidative stress caused by 5-fluorouracil can directly activate platelets, promoting thromboses[40]. Oxaliplatin-induced peripheral neuropathy can cause mobility problems, which are an important risk factor for VTE. In addition, oxaliplatin induces the release of pro-inflammatory cytokines and chemokines (e.g., IL-8, MCP-1), which attract leukocytes and promote a pro-thrombotic environment[41]. These chemotherapeutic agents also stimulate the release of TF-positive EVs (TF+ EVs) from tumor cells and endothelial cells (ECs), promoting coagulation[42]. Tamoxifen therapy against breast cancer can also cause VTE, likely because it decreases the levels of anticoagulant proteins, enhances platelet aggregation, and affects lipid metabolism[43]. Bevacizumab, a targeted vascular therapeutic agent for CRC, has also been associated with an increased thrombosis risk in oncology patients[44-46].
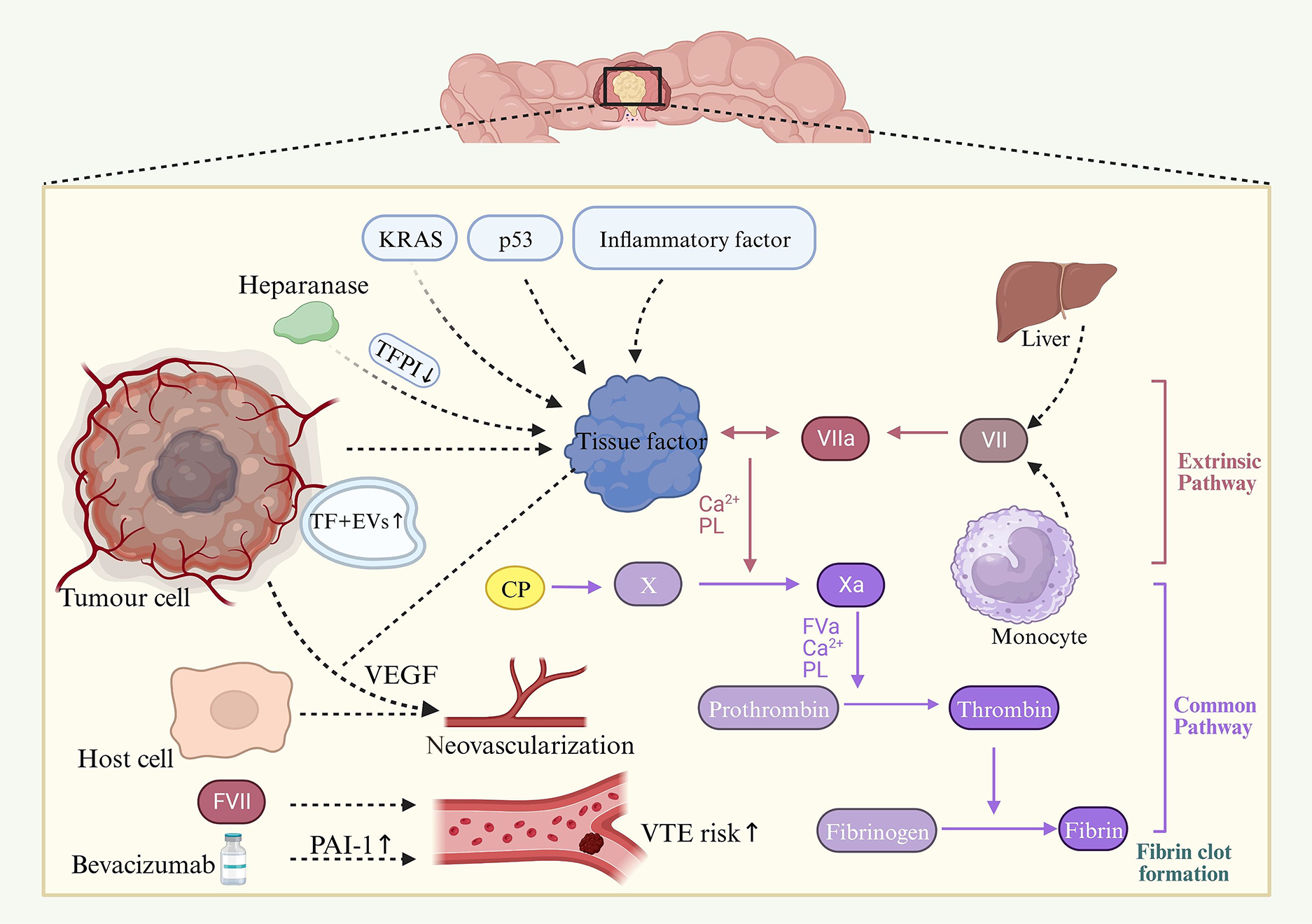
CRC cells actively contribute to the activation of the coagulation cascade. This activation is primarily driven by the expression of various procoagulant elements. Key among these are TF, CP, and a range of tumor-derived EVs. Additionally, CRC cells exhibit elevated levels of various coagulation factors and fibrinolytic proteins, further exacerbating the pro-thrombotic environment. Figure 2 shows a depiction of this complex interplay.
TF is widely regarded as a central player in CAT due to its significant roles in both tumor progression and VTE. As a primary initiator of blood coagulation under both normal and pathological conditions, TF, in conjunction with activated factor VII (FVII), initiates blood coagulation via the extrinsic pathway. Its expression is a common feature of numerous solid tumors and hematological malignancies. The TF expression level is often directly proportional to a tumor’s aggressiveness. Empirical evidence, particularly in colorectal and pancreatic cancers, suggests a correlation between plasma TF levels and tumor size[30,47]. Further, studies focusing on human CRCs indicate that TF positivity is associated with clinical stage, histological grade, a poor prognosis, and angiogenesis[48-50]. These findings underscore TF’s role not only in cancer-associated coagulopathies but also as a potential indicator, and possibly a determinant, of malignant tumor cell behavior. In lung cancers, particularly in non-small cell lung cancers, mutations in the epidermal growth factor receptor (EGFR) pathway upregulate TF expression and promote coagulation[51]. In addition, hypoxic lung tumors express hypoxia-inducible factors, and these in turn upregulate procoagulant factors, which may differ from those in CRC[52]. Additionally, the high inflammatory status of patients with CRC further boosts TF production[53]. The levels of inflammatory cytokines such as IL-6, TNF-α, and IL-1β are relatively lower in CRC than in pancreatic cancer, leading to a more pronounced prothrombotic milieu in pancreatic cancer[54]. The involvement of tumor cell-derived TF extends beyond its role in thrombin generation within the cancer milieu; TF also influences its own expression in both malignant and host vascular cells. It promotes tumor progression by upregulating the expression of vascular endothelial growth factor (VEGF). Another distinct procoagulant in tumor cells is CP, which activates factor X independently of FVII. The extent to which CP interacts with TF remains to be fully elucidated[55]. Moreover, CRC tissues express acetylheparinase, which promotes tumor progression by increasing TF expression and interacting with TF pathway inhibitors (TFPI) on the surface of endothelial and tumor cells. This interaction leads to the dissociation of TFPI, thereby augmenting cell surface TF activity[56].
Tumor-derived EVs, which carry lipids, proteins, and nucleic acids, are actively secreted by a diverse array of tumor cells during processes such as activation, apoptosis, or malignant transformation[57]. These EVs have garnered significant interest in the context of prethrombotic disorders due to their increased numbers and thrombogenic activity, highlighting their critical role in thrombosis pathogenesis[58]. Particularly noteworthy in tumor tissues are TF+ EVs. The high concentration of negatively charged phospholipids on the surface of these EVs significantly enhances their TF activity. Studies have established that the procoagulant activity of tumor-derived EVs is primarily due to the presence of TF[59,60]. Elevated levels of TF+ EVs have been identified in CRCs; however, their activity varies from that observed in patients with pancreatic cancer[42]. In addition, TF+ EVs have been shown to lead to the establishment of a thrombotic state in patients with cancer[61-63]. Wang et al[64] showed that only TF-positive tumor-bearing mice had elevated levels of TF+ EVs and enhanced thrombosis in the saphenous vein FeCl3 injury model. A recent meta-analysis including six studies concluded that TF-containing EVs are associated with an increased risk of VTE in cancer patients[65]. Microsatellite Instability high CRC is characterized by its strong immune response, leading to increased production of inflammatory cytokines and increased release of procoagulant EVs[66]. In addition, microRNAs (miRNAs) contained in EVs have important biological functions, and they can influence gene expression in distal cells. A recent nested case-control study addressed the link between miRNA expression and thrombogenesis in CRC. The authors compared the tumor expression miRNA profiles of patients with CRC with and without VTE. The primary results suggested differential expressions of 19 tumor miRNAs in VTE cases compared with controls, with hsa-miR-3652, hsa-miR-92b-5p, and hsa-miR-10394-5p being the most significantly downregulated in the patients with VTE[67]. The miRNA profile of tumor cells correlated with CAT and may contribute to the hypercoagulability observed in CRC. Another study showed that the generation of EVs is dependent on oncogenes. The authors found that cells overexpressing the MYC and AURKB genes release significantly more EVs than control cells. They also found that an inverse relationship between MYC upregulation and RAS/MEK/ERK signaling pathway activation regulates the release of EVs[68]. The potential use of EVs as predictive biomarkers for VTE risk in patients with cancer remains a subject of ongoing investigation. Current clinical trials are assessing the viability of measuring TF+ EVs as VTE predictors in patients with cancer[69]. Given the evident role of EVs in both thrombosis and cancer progression, exploring ways to modulate their release and activity could hold substantial clinical importance.
Under physiological conditions, plasma coagulation FVII is predominantly synthesized in the liver, primarily by hepatocytes[70]. However, FVII expression extends beyond hepatic production. It can also occur in monocytes within cancerous tissues and under inflammatory disease contexts[71]. This ectopic expression of FVII has been particularly observed in digestive tract tumors[72]. For instance, studies have consistently shown endogenous expression of FVII in colon carcinoma cell lines[73,74]. Ectopic FVII molecules are functional, partly due to the expression of c-glutamyl carboxylase in cancer cells, an enzyme that facilitates the necessary post-translational modifications for proper localization of FVII at the cell membrane[74]. Coagulation factor VIII (FVIII) is another crucial component of the coagulation cascade. Its elevated activity has been linked to increased risks of both primary and recurrent VTEs[75,76]. Patients with CRC have been reported to present high FVIII levels[77]. Results of a retrospective study showed higher FVIII levels in patients with cancer with a history of thrombosis than in matched controls without thrombosis[78]. This finding was corroborated in a prospective cohort study, which demonstrated that highly expressed FVIII plasma levels were a significant risk factor for VTE in patients with cancer[79]. Despite this evidence for the correlation between VTE risk and FVIII levels, the exact impact of malignant tumors on FVIII plasma levels warrants further investigation. Tumor cells seem to influence the host fibrinolytic system, they express fibrinolytic proteins and interact with their inhibitors including plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator inhibitor-2. PAI-1 inhibits fibrinolysis and has been associated with an increased thrombosis risk[80]. In a CRC mouse model, administration of bevacizumab, an anti-VEGF drug, led to increased levels of PAI-1 and thromboses. This effect was alleviated by the use of a PAI-1 inhibitor[81]. Additionally, a study in patients with pancreatic cancer suggested that high PAI-1 antigen and activity levels could predispose to VTE[82].
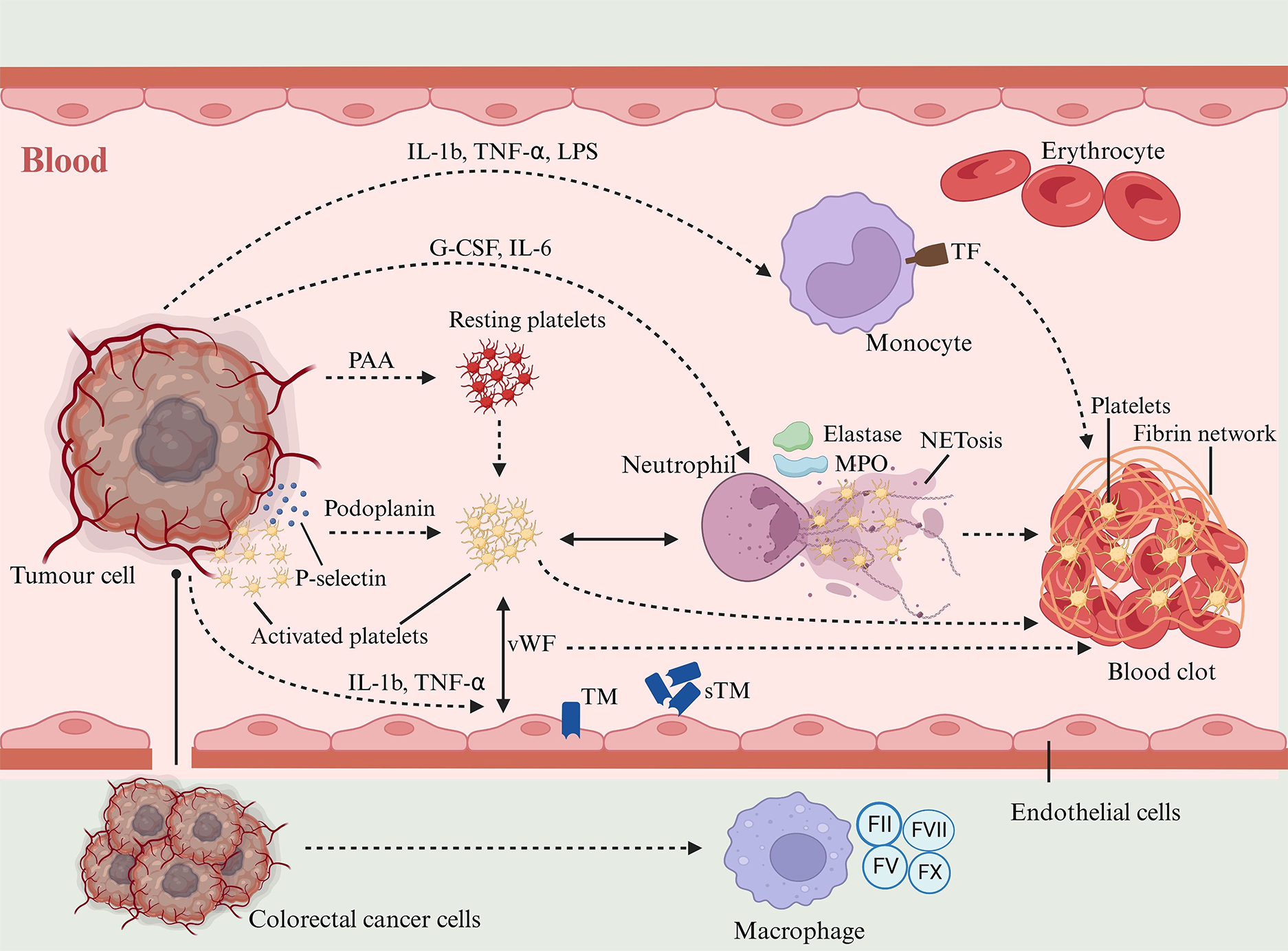
In CRC, interactions between tumor cells and host cells are mediated by two distinct mechanisms: Direct adhesions and secretion of soluble mediators. These interactions produce significant changes in the host cells, leading to the induction of a procoagulant phenotype. Figure 3 depicts the complexities of these interactions and their impact on host cell behavior.
Substantial evidence supports the role of platelets in fostering a hypercoagulable state in patients with CRC[83]. Malignant tumors induce platelet adhesion and aggregation either through direct cancer cell-platelet interactions or via tumor cell-secreted platelet aggregation stimulants, including ADP, thrombin, matrix metalloproteinases, and IL-6[21,84,85]. Elevated circulating levels of soluble P-selectin, correlated with higher VTE rates, have been observed in patients with cancer[86]. During the adhesion process, platelets express P-selectin, which binds tumor cells, forming aggregates and facilitating tumor proliferation and metastasis[87]. This interaction accelerates tumor growth and shields tumor cells from immune surveillance. Platelets aggregate around tumor cells, forming a ‘platelet cloak’ that effectively conceals them from natural killer cells[88]. The overproduction of mucins also generates a physical and biochemical barrier that protects tumor cells from recognition and attack by immune cells[89]. Mucin binds to P-selectin on platelets and promotes platelet adhesion, an interaction mechanism that may further consolidate the barrier function of tumor cells and lead to a more efficient immune escape[90]. MUC4 enhances the survival and extravasation of disseminated tumor cells by interacting with platelets[91]. Colorectal tumor cells might also harness Podoplanin, a sialoglycoprotein located on cell membranes, which can activate platelets[92]. Podoplanin, a ligand for the platelet receptor CLEC-2, induces platelet aggregation, a process intimately linked with tumor metastasis and malignancy progression[93]. Moreover, platelets interact with ECs, contributing to CAT. This was evidenced in a deep vein thrombosis mouse model, where platelets were shown to enhance blood hypercoagulability through interaction with endothelial-bound von Willebrand factor (vWF)[94]. Lastly, platelets facilitate thrombosis by activating the coagulation cascade, leading to thrombin generation. This occurs by the exposure of PS on their outer membrane, which serves as a platform for initiating fibrin clot formation[95].
Leukocyte counts are commonly high in patients with CRC, and this increase is associated with a high VTE risk[96]. Leukocytes, particularly neutrophils and monocytes, are significantly influenced by tumor cells, adopting a procoagulant phenotype. These cells are activated by direct contact with tumor cells or via the release of inflammatory cytokines into the bloodstream. CRC cells secrete factors like granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor, and IL-6, elevating the circulating levels[97]. G-CSF increases neutrophil counts and stimulates their activation[17]. Activated neutrophils release various procoagulant enzymes such as elastase, cathepsin G, and myeloperoxidase (MPO)[98].
Activated platelets in patients with CRC have a pivotal function in the development of hypercoagulability, interacting with various host cells. These interactions include stimulating leukocytes to release neutrophil extracellular traps (NETs), which are implicated in venous thromboses[99]. NETs, composed of desmosomal chromatin and proteins like neutrophil elastase (NE), tissue protease G, and MPO, were initially identified as a defense mechanism against pathogens[100-102]. However, in the context of CRC, NETs contribute to a prothrombotic state and thromboses vis multiple pathways[103,104]. NETs provide a structural scaffold for platelet and fibrin adhesion, aiding thrombus formation. Moreover, NETs components such as NE and cathepsin G can activate the coagulation pathway, promoting clotting[105]. Moreover, NETs capture and activate platelets, fostering a procoagulant phenotype and accelerating development of thrombi[106]. Zhang et al[107] provided significant insights into the role of NETs in CRC thromboses. Their study, comparing 60 patients with CRC with 20 healthy controls, revealed a higher prevalence of NETs in patients with cancer, correlating with disease progression.
The role of activated monocytes and macrophages in CAT is well-documented. Macrophages infiltrating tumors adopt a locally activated, procoagulant state, which contributes to the deposition of fibrin within tumor tissues[108]. A study on advanced CRC demonstrated that blood monocytes with a procoagulant phenotype increase the risk of intravascular coagulation and thromboembolic complications[109]. Monocytes distinguish themselves from other circulating blood cells through their capacity to synthesize and express procoagulant TFs upon activation. This activation is triggered by cytokines like IL-1β, TNF-α, and lipopolysaccharide on their surface[110]. These mediators, often secreted by cancer cells, trigger monocyte-mediated thrombotic mechanisms. Additionally, tumor-infiltrating macrophages have been identified as expressing coagulation factors II, V, VII, and X, further implicating them in CAT. Recent research has expanded this understanding by showing that blood monocytes can release extracellular traps (ECTs) in response to various inflammatory stimuli[111]. However, the exact role of these ECTs in the context of CAT, remains unclear.
Under normal physiological conditions, ECs maintain blood flow by providing a smooth, antithrombotic surface that prevents platelet adhesion and coagulation. In patients with CRC, several factors disrupt this equilibrium. Tumor cells can activate ECs either through direct cell-to-cell contact, as observed in CRC cases, or by releasing inflammatory mediators and acute-phase proteins that stimulate endothelial activation[112,113]. Cytokines like IL-1b and TNF-α are key regulators altering ECs functions related to hemostasis, by altering the expression of thrombomodulin (TM), TF, vWF, selectins, and fibrinolytic proteins[114]. TM, an endothelial membrane receptor with significant anticoagulant properties, forms a complex with thrombin to activate protein C, a natural anticoagulant[115]. However, in patients with CRC, an increase in soluble TM levels and a decrease in surface TM expression mark the loss of the endothelial anticoagulant function[116]. Under inflammation, TNF-α from ECs stimulates the expression and release of soluble TF, a potent prothrombotic agent. The shift from an anticoagulant to a prothrombotic endothelium in CRC is marked by the upregulated procoagulant TF and the downregulated TM/protein C system[117]. In addition, activated ECs release soluble adhesion molecules like E-selectin and P-selectin, which have been found at elevated levels in patients with CRC with thrombosis[118]. These molecules, particularly P-selectin, enhance VTE by recruiting leukocytes and promoting platelet adhesion and aggregation, thereby increasing the thrombotic risk[119]. Moreover, endothelial-derived nitric oxide, an inhibitor of platelet adhesion and aggregation, is reduced in CRC, further contributing to the thrombotic milieu[120]. Lastly, while the counts of circulating ECs are elevated during endothelial injury in solid tumors, their specific role in CAT remains unclear[121].
Molecular biology research has increasingly clarified the function of oncogenes in tumor transformation and their impact on the expression of coagulation-related proteins within cancerous tissues. These processes occur after mutations and deletions in genes like KRAS, EGFR, MET, PTEN, and TP53. Historically, hypercoagulable states and thrombosis in patients with cancer were perceived as nonspecific byproducts of cancer progression and associated vascular disruptions, often attributed to vascular hyperpermeability and inflammation[13]. However, emerging evidence now points to these coagulopathies being cancer-specific phenomena[13]. Rak and colleagues have argued that the coagulation system’s functionality is altered by different types of cancer cell, noting significant variations in VTE risk across different cancers. For instance, CRCs display a higher VTE risk than skin, breast, and prostate cancers[29,122]. This suggests that specific tumor genotypes might directly influence the coagulation system or induce changes in the tumor microenvironment[123]. Additionally, these pathways can directly dysregulate coagulation effectors via different mechanisms: The aberrant expression of TFs, the induction of ectopic coagulation genes, or the release of EVs containing TF-positive genes into the systemic circulation[124,125].
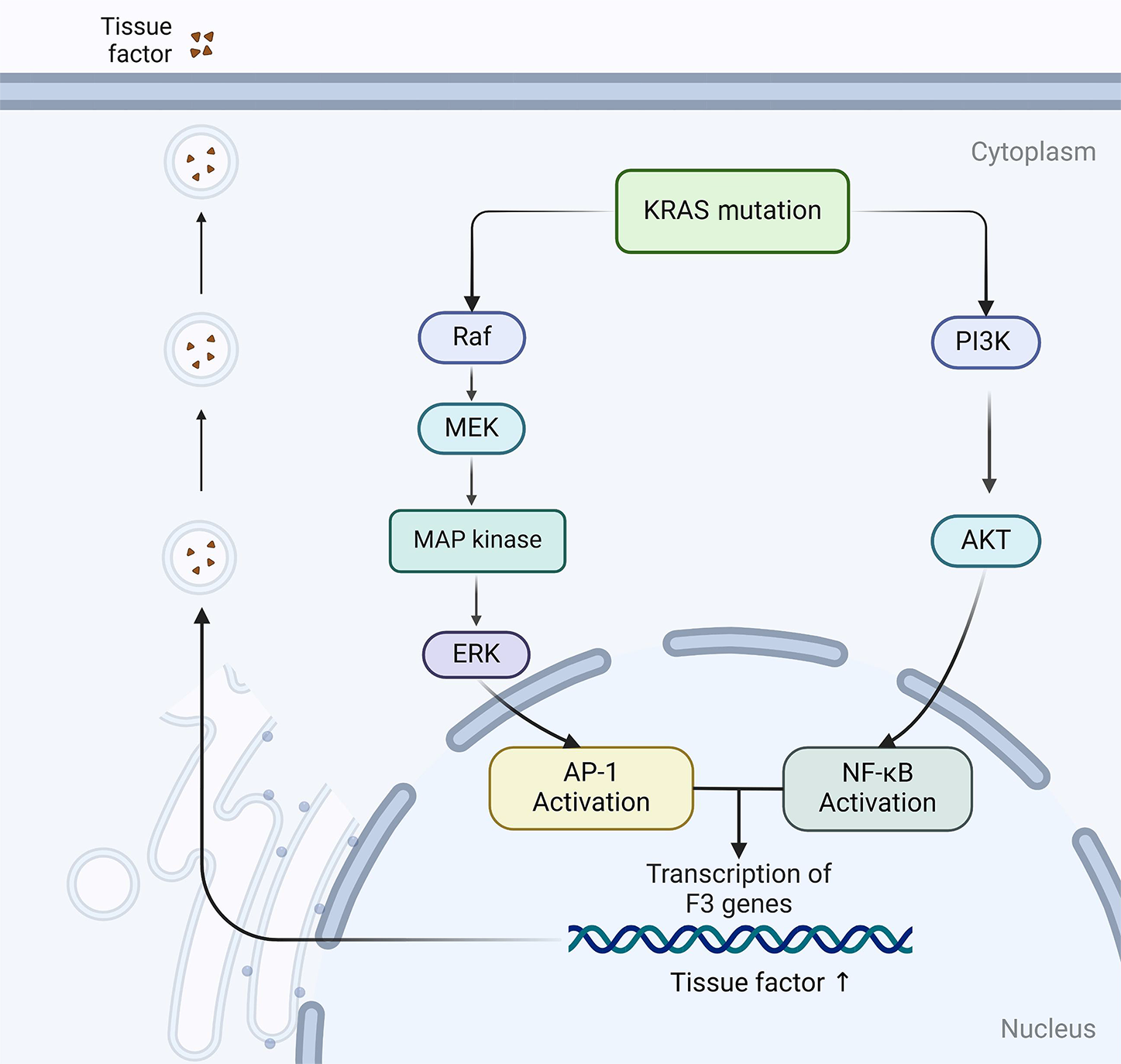
Yu et al[47] have produced evidence showing the role of TF expression in human CRC cells. Their results delineate how TF expression is regulated by two pivotal translational events that mark the progression of this disease: Activation of the KRAS oncogene and inactivation of the p53 oncogene. Intriguingly, this regulation is contingent upon the activity of the MEK/mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K) pathways. These findings implicate the MAPK and PI3K pathways as potential participants in the upregulation of TF in CRC cells, which lead to a high VTE risk. Further corroborating this, Regina et al[126] validated these observations in actual CRC samples. Beyond the scope of KRAS, mutations in PTEN are also implicated in the increased expression of TF, adding another layer of complexity to this regulatory mechanism[127]. Additionally, less expected genetic variants have been explored to explain the development of VTE among patients with cancer. A notable study revealed that patients with CRC with the β3-integrin rs3809865 A/A genotype faced a heightened risk of VTE than those with A/T or T/T genotypes[128]. KRAS mutations are present in approximately 40% of patients with CRC, usually occurring at codons 12, 13, and 61, resulting in constitutive activation of the RAS/MAPK and PI3K/AKT signaling pathways. Experimental studies have shown that mutant KRAS proteins promote EC activation and upregulate TF expression through multiple mechanisms. Specifically, oncogenic KRAS induces activation of the RAF-MEK-ERK and PI3K-AKT pathways, leading to enhanced transcription of TF through AP-1 and NF-kB signaling (Figure 4)[129,130]. In addition, KRAS mutations increase the secretion of VEGF, stimulating endothelial TF expression and promoting the release of procoagulant EVs from tumor cells, thereby amplifying the coagulation cascade response[131]. Clinical evidence supports this mechanism as patients with metastatic CRC harboring KRAS mutations have a significantly increased risk of VTE (OR, 2.21; 95%CI: 1.08-4.53), reinforcing the role of KRAS-driven hypercoagulability[132]. Although patients with BRAF mutations in CRC have also exhibited high rates of VTE, the number of cases has been insufficient to definitively establish a role for BRAF as a risk factor for VTE[133].
The coagulation phenotype of cancer cells is increasingly recognized as a key mechanism bridging the genetic evolution of the disease with its biological and clinical characteristics[8]. Research has shed light on the heightened incidence of CRC in individuals predisposed to certain thrombotic conditions, particularly those with a factor V Leiden mutation[134]. This emerging evidence proposes a change in basic assumptions: Procoagulant events in CRC may not merely be concurrent phenomena, they may be active contributors to tumor growth and progression, and potentially, initiators of malignant transformation. From a therapeutic standpoint, this revelation underscores the potential of targeting coagulation disturbances for cancer management. The refinement of strategies to control coagulation perturbations could pave the way for novel approaches in the treatment, control, and prevention of cancer. Such strategies may mitigate the direct impacts of cancer and also impede its progression by addressing underlying coagulation-related mechanisms.
The discovery that coagulation events can be genetically driven is important. The correlation of KRAS mutations in colorectal and lung cancers with heightened VTE risks stands in contrast to the IDH1/2 mutations in glioblastomas, which appear to reduce the VTE risk[132,135,136]. Given the labor-intensive work of examining each gene’s role in CAT, a more efficient approach might involve an unbiased genomic exploration. This strategy could unveil novel biomarkers and potentially reveal new therapeutic targets for managing CAT. Moreover, a comprehensive understanding of the primary biological mechanisms that precipitate hypercoagulability and consequent VTE necessitates an in-depth evaluation of the genetic landscapes specific to each cancer subtype. This approach is crucial, as the genetic determinants associated with CAT are likely to vary according to the unique processes inherent to each cancer type. Such targeted investigations promise to identify patient subgroups with high VTE risks, enabling the development of precise and effective anticoagulation strategies. This, in turn, may significantly improve thrombosis prevention in patients with cancer, marking a critical step forward in managing this complex and challenging clinical issue.
In this article, we systematically reviewed the molecular and clinical mechanisms of CAT in patients with CRC, focusing on the procoagulant properties of tumor cells themselves and their interactions with host cells. In the realm of CRC, the challenge posed by CAT is substantial, given its significant contribution to increased morbidity and mortality. Despite years of dedicated research aimed at unraveling the mechanisms underlying CAT and identifying high-risk patient profiles, advancements have been gradual. Significant progress in the treatment of CAT in CRC will be made with the development of personalized medical approaches that integrate newly discovered biomarkers and targeted therapies. Emerging biomarkers such as CTCs and tumor-derived EVs are expected to enable precise risk stratification and monitoring. In terms of prevention strategies, regular monitoring of coagulation function and the use of thrombosis risk assessment tools can help clinicians implement targeted preventive anticoagulant therapy. On the therapeutic side, the development and clinical integration of novel oral anticoagulants, such as rivaroxaban and edoxaban, are expected to significantly improve the therapeutic efficacy of conventional anticoagulants, such as low molecular weight heparin and warfarin. Studies focusing on the tumor microenvironment have revealed interactions between cancer cells and the coagulation system. TF expression is frequently upregulated in CRC and may be a new therapeutic target. In addition, the integration of artificial intelligence and machine learning with large-scale clinical and genomic data is expected to revolutionize predictive models for CAT. Future studies will also explore the role of immunotherapy and anti-inflammatory treatments and their interactions on cancer progression with thrombotic events. These advances will pave the way for a comprehensive multidisciplinary approach addressing CAT and improving the quality of life and survival of patients with CRC.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 556] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 3. | Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 4. | Falanga A, Russo L. Epidemiology, risk and outcomes of venous thromboembolism in cancer. Hamostaseologie. 2012;32:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Heit JA, O'Fallon WM, Petterson TM, Lohse CM, Silverstein MD, Mohr DN, Melton LJ 3rd. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 776] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 6. | Streiff MB. Thrombosis in the setting of cancer. Hematology Am Soc Hematol Educ Program. 2016;2016:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1459] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 8. | Blom JW, Vanderschoot JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 477] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 9. | Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1059] [Cited by in RCA: 1169] [Article Influence: 64.9] [Reference Citation Analysis (1)] |
| 10. | Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 841] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 11. | Thaler J, Ay C, Pabinger I. Venous thromboembolism in cancer patients - risk scores and recent randomised controlled trials. Thromb Haemost. 2012;108:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Khalil J, Bensaid B, Elkacemi H, Afif M, Bensaid Y, Kebdani T, Benjaafar N. Venous thromboembolism in cancer patients: an underestimated major health problem. World J Surg Oncol. 2015;13:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Magnus N, D'Asti E, Meehan B, Garnier D, Rak J. Oncogenes and the coagulation system--forces that modulate dormant and aggressive states in cancer. Thromb Res. 2014;133 Suppl 2:S1-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Nakagawa K, Watanabe J, Suwa Y, Suzuki S, Ishibe A, Ota M, Kunisaki C, Endo I. Clinical analysis of preoperative deep vein thrombosis risk factors in patients with colorectal cancer: Retrospective observational study. Ann Gastroenterol Surg. 2019;3:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Wei Q, Wei ZQ, Jing CQ, Li YX, Zhou DB, Lin MB, He XL, Li F, Liu Q, Zheng JY, Wang GY, Tu SL, Wang ZJ, Li A, Xiao G, Zhuang J, Bai L, Huang H, Li Y, Song W, Liang ZL, Shen ZL, Liu FL, Dai Y, Zhou XJ, Dong M, Wang H, Qiu J, Zhou L, Li XX, Wang ZQ, Zhang H, Wang Q, Pang MH, Wei HB, Hu ZQ, Yan YD, Che Y, Gu ZC, Yao HW, Zhang ZT; and for the CRC-VTE investigators. Incidence, prevention, risk factors, and prediction of venous thromboembolism in Chinese patients after colorectal cancer surgery: a prospective, multicenter cohort study. Int J Surg. 2023;109:3003-3012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 16. | Griffith KA, Ryan AS. IL-6 and Soluble Receptors in Overweight and Obese African American Women With and Without Breast Cancer. Biol Res Nurs. 2021;23:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Demers M, Wagner DD. Neutrophil extracellular traps: A new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology. 2013;2:e22946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Simanek R, Vormittag R, Ay C, Alguel G, Dunkler D, Schwarzinger I, Steger G, Jaeger U, Zielinski C, Pabinger I. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost. 2010;8:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 19. | Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822-2829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 411] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 20. | Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1530] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 21. | Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33:231-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 22. | Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, Soff G, Parameswaran R, Hassoun H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466-3473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 23. | Ay C, Dunkler D, Marosi C, Chiriac AL, Vormittag R, Simanek R, Quehenberger P, Zielinski C, Pabinger I. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377-5382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 563] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 24. | Jick H, Slone D, Westerholm B, Inman WH, Vessey MP, Shapiro S, Lewis GP, Worcester J. Venous thromboembolic disease and ABO blood type. A cooperative study. Lancet. 1969;1:539-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 210] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Bafunno V, Margaglione M. Genetic basis of thrombosis. Clin Chem Lab Med. 2010;48 Suppl 1:S41-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Yang SS, Yu CS, Yoon YS, Yoon SN, Lim SB, Kim JC. Symptomatic venous thromboembolism in Asian colorectal cancer surgery patients. World J Surg. 2011;35:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | McLeod RS, Geerts WH, Sniderman KW, Greenwood C, Gregoire RC, Taylor BM, Silverman RE, Atkinson KG, Burnstein M, Marshall JC, Burul CJ, Anderson DR, Ross T, Wilson SR, Barton P; Canadian Colorectal Surgery DVT Prophylaxis Trial investigators. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the canadian colorectal DVT prophylaxis trial: a randomized, double-blind trial. Ann Surg. 2001;233:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339-2346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 593] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 29. | Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22:9-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Muscat-Baron L, Borg AL, Attard LM, Gatt A, Riva N. Cancer-Associated Abdominal Vein Thrombosis. Cancers (Basel). 2023;15:5293. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Citla Sridhar D, Abou-Ismail MY, Ahuja SP. Central venous catheter-related thrombosis in children and adults. Thromb Res. 2020;187:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 33. | Shao B, Wahrenbrock MG, Yao L, David T, Coughlin SR, Xia L, Varki A, McEver RP. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood. 2011;118:4015-4023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Wahrenbrock M, Borsig L, Le D, Varki N, Varki A. Selectin-mucin interactions as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. J Clin Invest. 2003;112:853-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Woei-A-Jin FJ, Tesselaar ME, Garcia Rodriguez P, Romijn FP, Bertina RM, Osanto S. Tissue factor-bearing microparticles and CA19.9: two players in pancreatic cancer-associated thrombosis? Br J Cancer. 2016;115:332-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 422] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 37. | Kawano T, Englisch C, Hisada Y, Paul D, Archibald S, Grover S, Pabinger I, Ay C, Mackman N. Mucin 1 and venous thrombosis in tumor-bearing mice and patients with cancer. Thromb Res. 2024;237:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 38. | Falanga A, Marchetti M. Anticancer treatment and thrombosis. Thromb Res. 2012;129:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Lechner D, Weltermann A. Chemotherapy-induced thrombosis: a role for microparticles and tissue factor? Semin Thromb Hemost. 2008;34:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol Ther. 2020;206:107447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 41. | Kang L, Tian Y, Xu S, Chen H. Oxaliplatin-induced peripheral neuropathy: clinical features, mechanisms, prevention and treatment. J Neurol. 2021;268:3269-3282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 42. | Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 43. | Kirwan CC, Blower EL. Contemporary breast cancer treatment-associated thrombosis. Thromb Res. 2022;213 Suppl 1:S8-S15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 44. | Stathopoulos GP, Batziou C, Trafalis D, Koutantos J, Batzios S, Stathopoulos J, Legakis J, Armakolas A. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology. 2010;78:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol. 2007;14:1860-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 46. | Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008;300:2277-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 571] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 47. | Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 425] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 48. | Seto S, Onodera H, Kaido T, Yoshikawa A, Ishigami S, Arii S, Imamura M. Tissue factor expression in human colorectal carcinoma: correlation with hepatic metastasis and impact on prognosis. Cancer. 2000;88:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 49. | Shigemori C, Wada H, Matsumoto K, Shiku H, Nakamura S, Suzuki H. Tissue factor expression and metastatic potential of colorectal cancer. Thromb Haemost. 1998;80:894-898. [PubMed] |
| 50. | Nakasaki T, Wada H, Shigemori C, Miki C, Gabazza EC, Nobori T, Nakamura S, Shiku H. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol. 2002;69:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Yang S, Yang L, Wu Y, Zhang C, Wang S, Ma N, Wang L, Wang Q. Anaplastic lymphoma kinase rearrangement may increase the incidence of venous thromboembolism by increasing tissue factor expression in advanced lung adenocarcinoma. Ann Transl Med. 2020;8:1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Evans CE, Bendahl PO, Belting M, Branco C, Johnson RS. Diverse roles of cell-specific hypoxia-inducible factor 1 in cancer-associated hypercoagulation. Blood. 2016;127:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer. 2003;2:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 54. | Farajzadeh Valilou S, Keshavarz-Fathi M, Silvestris N, Argentiero A, Rezaei N. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev. 2018;39:46-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 55. | Szajda SD, Darewicz B, Gorzel M, Zalewska B, Skrzydlewski Z, Kudelski J, Domel T. [Cancer procoagulant (CP)]. Przegl Lek. 2005;62:169-172. [PubMed] |
| 56. | Nadir Y, Brenner B. Heparanase procoagulant activity in cancer progression. Thromb Res. 2016;140 Suppl 1:S44-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Popēna I, Ābols A, Saulīte L, Pleiko K, Zandberga E, Jēkabsons K, Endzeliņš E, Llorente A, Linē A, Riekstiņa U. Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun Signal. 2018;16:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 58. | Lima LG, Leal AC, Vargas G, Porto-Carreiro I, Monteiro RQ. Intercellular transfer of tissue factor via the uptake of tumor-derived microvesicles. Thromb Res. 2013;132:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 60. | Hisada Y, Mackman N. Tissue Factor and Extracellular Vesicles: Activation of Coagulation and Impact on Survival in Cancer. Cancers (Basel). 2021;13:3839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 61. | Bollen L, Peetermans M, Peeters M, Van Steen K, Hoylaerts MF, Declerck PJ, Verhamme P, Gils A. Active PAI-1 as marker for venous thromboembolism: case-control study using a comprehensive panel of PAI-1 and TAFI assays. Thromb Res. 2014;134:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Meltzer ME, Lisman T, de Groot PG, Meijers JC, le Cessie S, Doggen CJ, Rosendaal FR. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 63. | Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105:1102-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 64. | Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543-5552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 65. | Cui CJ, Wang GJ, Yang S, Huang SK, Qiao R, Cui W. Tissue Factor-bearing MPs and the risk of venous thrombosis in cancer patients: A meta-analysis. Sci Rep. 2018;8:1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 66. | Russo G, Pepe F, Pisapia P, Palumbo L, Nacchio M, Vigliar E, Pallante P, Parente P, Fassan M, Graziano P, Bellevicine C, Troncone G, Malapelle U, Iaccarino A. Microsatellite instability evaluation of patients with solid tumour: routine practice insight from a large series of Italian referral centre. J Clin Pathol. 2023;76:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 67. | Anijs RJS, Laghmani EH, Ünlü B, Kiełbasa SM, Mei H, Cannegieter SC, Klok FA, Kuppen PJK, Versteeg HH, Buijs JT. Tumor-expressed microRNAs associated with venous thromboembolism in colorectal cancer. Res Pract Thromb Haemost. 2022;6:e12749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Kilinc S, Paisner R, Camarda R, Gupta S, Momcilovic O, Kohnz RA, Avsaroglu B, L'Etoile ND, Perera RM, Nomura DK, Goga A. Oncogene-regulated release of extracellular vesicles. Dev Cell. 2021;56:1989-2006.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 69. | Zwicker JI. Predictive value of tissue factor bearing microparticles in cancer associated thrombosis. Thromb Res. 2010;125 Suppl 2:S89-S91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 70. | Strickland DK, Au DT, Cunfer P, Muratoglu SC. Low-density lipoprotein receptor-related protein-1: role in the regulation of vascular integrity. Arterioscler Thromb Vasc Biol. 2014;34:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 71. | Zelaya H, Grunz K, Nguyen TS, Habibi A, Witzler C, Reyda S, Gonzalez-Menendez I, Quintanilla-Martinez L, Bosmann M, Weiler H, Ruf W. Nucleic acid sensing promotes inflammatory monocyte migration through biased coagulation factor VIIa signaling. Blood. 2024;143:845-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 72. | Neaud V, Hisaka T, Monvoisin A, Bedin C, Balabaud C, Foster DC, Desmoulière A, Kisiel W, Rosenbaum J. Paradoxical pro-invasive effect of the serine proteinase inhibitor tissue factor pathway inhibitor-2 on human hepatocellular carcinoma cells. J Biol Chem. 2000;275:35565-35569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Tang JQ, Fan Q, Wu WH, Jia ZC, Li H, Yang YM, Liu YC, Wan YL. Extrahepatic synthesis of coagulation factor VII by colorectal cancer cells promotes tumor invasion and metastasis. Chin Med J (Engl). 2010;123:3559-3565. [PubMed] |
| 74. | Koizume S, Jin MS, Miyagi E, Hirahara F, Nakamura Y, Piao JH, Asai A, Yoshida A, Tsuchiya E, Ruf W, Miyagi Y. Activation of cancer cell migration and invasion by ectopic synthesis of coagulation factor VII. Cancer Res. 2006;66:9453-9460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 75. | Cristina L, Benilde C, Michela C, Mirella F, Giuliana G, Gualtiero P. High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. Br J Haematol. 2004;124:504-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Kyrle PA, Minar E, Hirschl M, Bialonczyk C, Stain M, Schneider B, Weltermann A, Speiser W, Lechner K, Eichinger S. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000;343:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 502] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 77. | Battistelli S, Stefanoni M, Lorenzi B, Dell'avanzato R, Varrone F, Pascucci A, Petrioli R, Vittoria A. Coagulation factor levels in non-metastatic colorectal cancer patients. Int J Biol Markers. 2008;23:36-41. [PubMed] |
| 78. | Dogan M, Demirkazik A, Konuk N, Yalcin B, Buyukcelik A, Utkan G, Tek I, Akbulut H, Sencan O, Icli F. The effect of venous thromboembolism on survival of cancer patients and its relationship with serum levels of factor VIII and vascular endothelial growth factor: a prospective matched-paired study. Int J Biol Markers. 2006;21:206-210. [PubMed] |
| 79. | Vormittag R, Simanek R, Ay C, Dunkler D, Quehenberger P, Marosi C, Zielinski C, Pabinger I. High factor VIII levels independently predict venous thromboembolism in cancer patients: the cancer and thrombosis study. Arterioscler Thromb Vasc Biol. 2009;29:2176-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 80. | Westrick RJ, Eitzman DT. Plasminogen activator inhibitor-1 in vascular thrombosis. Curr Drug Targets. 2007;8:966-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Chen N, Ren M, Li R, Deng X, Li Y, Yan K, Xiao L, Yang Y, Wang L, Luo M, Fay WP, Wu J. Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol Cancer. 2015;14:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 83. | Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014;41:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Lee EC, Cameron SJ. Cancer and Thrombotic Risk: The Platelet Paradigm. Front Cardiovasc Med. 2017;4:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Falanga A, Russo L, Verzeroli C. Mechanisms of thrombosis in cancer. Thromb Res. 2013;131 Suppl 1:S59-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 86. | Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C, Pabinger I. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008;112:2703-2708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 320] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 87. | Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 329] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 88. | Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 747] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 89. | Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A, Banerjee K, Jain M, Solheim JC, Kumar S, Batra SK. Cancer-associated mucins: role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 90. | Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci U S A. 2001;98:3352-3357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 533] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 91. | Rowson-Hodel AR, Wald JH, Hatakeyama J, O'Neal WK, Stonebraker JR, VanderVorst K, Saldana MJ, Borowsky AD, Sweeney C, Carraway KL 3rd. Membrane Mucin Muc4 promotes blood cell association with tumor cells and mediates efficient metastasis in a mouse model of breast cancer. Oncogene. 2018;37:197-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 92. | Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008;28:2997-3006. [PubMed] |
| 93. | Riedl J, Preusser M, Nazari PM, Posch F, Panzer S, Marosi C, Birner P, Thaler J, Brostjan C, Lötsch D, Berger W, Hainfellner JA, Pabinger I, Ay C. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129:1831-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 94. | Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Köllnberger M, Wakefield TW, Lämmle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 366] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 95. | Vanschoonbeek K, Feijge MA, Van Kampen RJ, Kenis H, Hemker HC, Giesen PL, Heemskerk JW. Initiating and potentiating role of platelets in tissue factor-induced thrombin generation in the presence of plasma: subject-dependent variation in thrombogram characteristics. J Thromb Haemost. 2004;2:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Blix K, Jensvoll H, Brækkan SK, Hansen JB. White blood cell count measured prior to cancer development is associated with future risk of venous thromboembolism--the Tromsø study. PLoS One. 2013;8:e73447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 97. | Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92:2399-2405. [PubMed] [DOI] [Full Text] |
| 98. | Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122:2176-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 99. | Chang YW, Hsieh PW, Chang YT, Lu MH, Huang TF, Chong KY, Liao HR, Cheng JC, Tseng CP. Identification of a novel platelet antagonist that binds to CLEC-2 and suppresses podoplanin-induced platelet aggregation and cancer metastasis. Oncotarget. 2015;6:42733-42748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 100. | Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5773] [Cited by in RCA: 7224] [Article Influence: 344.0] [Reference Citation Analysis (0)] |
| 101. | Dąbrowska D, Jabłońska E, Garley M, Ratajczak-Wrona W, Iwaniuk A. New Aspects of the Biology of Neutrophil Extracellular Traps. Scand J Immunol. 2016;84:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 102. | Ravindran M, Khan MA, Palaniyar N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules. 2019;9:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 103. | Zhou Y, Tao W, Shen F, Du W, Xu Z, Liu Z. The Emerging Role of Neutrophil Extracellular Traps in Arterial, Venous and Cancer-Associated Thrombosis. Front Cardiovasc Med. 2021;8:786387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 104. | Jung HS, Gu J, Kim JE, Nam Y, Song JW, Kim HK. Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression. PLoS One. 2019;14:e0216055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 105. | Zhou Y, Xu Z, Liu Z. Impact of Neutrophil Extracellular Traps on Thrombosis Formation: New Findings and Future Perspective. Front Cell Infect Microbiol. 2022;12:910908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 106. | Li JC, Zou XM, Yang SF, Jin JQ, Zhu L, Li CJ, Yang H, Zhang AG, Zhao TQ, Chen CY. Neutrophil extracellular traps participate in the development of cancer-associated thrombosis in patients with gastric cancer. World J Gastroenterol. 2022;28:3132-3149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 107. | Zhang Y, Wang C, Yu M, Zhao X, Du J, Li Y, Jing H, Dong Z, Kou J, Bi Y, Novakovic VA, Zhou J, Shi J. Neutrophil extracellular traps induced by activated platelets contribute to procoagulant activity in patients with colorectal cancer. Thromb Res. 2019;180:87-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 108. | Gi T, Kuwahara A, Yamashita A, Matsuda S, Maekawa K, Moriguchi-Goto S, Sato Y, Asada Y. Histopathological Features of Cancer-Associated Venous Thromboembolism: Presence of Intrathrombus Cancer Cells and Prothrombotic Factors. Arterioscler Thromb Vasc Biol. 2023;43:146-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 109. | Laurent M, Joimel U, Varin R, Cazin L, Gest C, Le-Cam-Duchez V, Jin J, Liu J, Vannier JP, Lu H, Soria J, Li H, Soria C. Comparative study of the effect of rivaroxaban and fondaparinux on monocyte's coagulant activity and cytokine release. Exp Hematol Oncol. 2014;3:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 110. | Pabinger I, Posch F. Flamethrowers: blood cells and cancer thrombosis risk. Hematology Am Soc Hematol Educ Program. 2014;2014:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Granger V, Faille D, Marani V, Noël B, Gallais Y, Szely N, Flament H, Pallardy M, Chollet-Martin S, de Chaisemartin L. Human blood monocytes are able to form extracellular traps. J Leukoc Biol. 2017;102:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 112. | Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 113. | Tas F, Duranyildiz D, Argon A, Oğuz H, Camlica H, Yasasever V, Topuz E. Serum levels of leptin and proinflammatory cytokines in advanced-stage non-small cell lung cancer. Med Oncol. 2005;22:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34:107-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 115. | Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24:1374-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 116. | Kim JE, Yoo HJ, Gu JY, Kim HK. Histones Induce the Procoagulant Phenotype of Endothelial Cells through Tissue Factor Up-Regulation and Thrombomodulin Down-Regulation. PLoS One. 2016;11:e0156763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 118. | Karakantza M, Giannakoulas NC, Zikos P, Sakellaropoulos G, Kouraklis A, Aktypi A, Metallinos IC, Theodori E, Zoumbos NC, Maniatis A. Markers of endothelial and in vivo platelet activation in patients with essential thrombocythemia and polycythemia vera. Int J Hematol. 2004;79:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 119. | Meier TR, Myers DD Jr, Wrobleski SK, Zajkowski PJ, Hawley AE, Bedard PW, Ballard NE, Londy FJ, Kaila N, Vlasuk GP, Schaub RG, Wakefield TW. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 120. | Cella G, Marchetti M, Vianello F, Panova-Noeva M, Vignoli A, Russo L, Barbui T, Falanga A. Nitric oxide derivatives and soluble plasma selectins in patients with myeloproliferative neoplasms. Thromb Haemost. 2010;104:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 121. | Fleitas T, Martínez-Sales V, Vila V, Reganon E, Mesado D, Martín M, Gómez-Codina J, Montalar J, Reynés G. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One. 2012;7:e47365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 122. | Rak J, Klement G. Impact of oncogenes and tumor suppressor genes on deregulation of hemostasis and angiogenesis in cancer. Cancer Metastasis Rev. 2000;19:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Magnus N, Gerges N, Jabado N, Rak J. Coagulation-related gene expression profile in glioblastoma is defined by molecular disease subtype. J Thromb Haemost. 2013;11:1197-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 124. | Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, Montermini L, Kislinger T, Rak J. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287:43565-43572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 125. | Rickles FR. Cancer and thrombosis in women - molecular mechanisms. Thromb Res. 2009;123 Suppl 2:S16-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 126. | Regina S, Rollin J, Bléchet C, Iochmann S, Reverdiau P, Gruel Y. Tissue factor expression in non-small cell lung cancer: relationship with vascular endothelial growth factor expression, microvascular density, and K-ras mutation. J Thorac Oncol. 2008;3:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 127. | Mege D, Mezouar S, Dignat-George F, Panicot-Dubois L, Dubois C. Microparticles and cancer thrombosis in animal models. Thromb Res. 2016;140 Suppl 1:S21-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Bianconi D, Schuler A, Pausz C, Geroldinger A, Kaider A, Lenz HJ, Kornek G, Scheithauer W, Zielinski CC, Pabinger I, Ay C, Prager GW. Integrin beta-3 genetic variants and risk of venous thromboembolism in colorectal cancer patients. Thromb Res. 2015;136:865-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Galbusera M, Noris M, Remuzzi G. Thrombotic thrombocytopenic purpura--then and now. Semin Thromb Hemost. 2006;32:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 130. | Schwäble J, Choudhary C, Thiede C, Tickenbrock L, Sargin B, Steur C, Rehage M, Rudat A, Brandts C, Berdel WE, Müller-Tidow C, Serve H. RGS2 is an important target gene of Flt3-ITD mutations in AML and functions in myeloid differentiation and leukemic transformation. Blood. 2005;105:2107-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 131. | Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B. Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15:6830-6840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 384] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 132. | Ades S, Kumar S, Alam M, Goodwin A, Weckstein D, Dugan M, Ashikaga T, Evans M, Verschraegen C, Holmes CE. Tumor oncogene (KRAS) status and risk of venous thrombosis in patients with metastatic colorectal cancer. J Thromb Haemost. 2015;13:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 133. | Ortega Morán L, García Alfonso P, Aguilar Caballero I, Morón García B, Tirado Anula V, de Toro Carmena M, Soto Alsar J, Gutiérrez Alonso N, Bringas Beranek M, Martín Jiménez M, Muñoz Martín AJ. Incidence of venous thromboembolism in patients with colorectal cancer according to oncogenic status. Clin Transl Oncol. 2020;22:2026-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Vossen CY, Hoffmeister M, Chang-Claude JC, Rosendaal FR, Brenner H. Clotting factor gene polymorphisms and colorectal cancer risk. J Clin Oncol. 2011;29:1722-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 135. | Mandel JJ, Youssef M, Yust-Katz S, Patel AJ, Jalali A, Li Z, Wu J, Ludmir EB, de Groot JF. IDH mutation status and the development of venous thromboembolism in astrocytoma patients. J Neurol Sci. 2021;427:117538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 136. | Huang Y, Ding H, Luo M, Li S, Xie C, Zhong Y, Li Z. Combined analysis of clinical and laboratory markers to predict the risk of venous thromboembolism in patients with IDH1 wild-type glioblastoma. Support Care Cancer. 2022;30:6063-6069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |