Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.103892
Revised: February 5, 2025
Accepted: March 26, 2025
Published online: April 14, 2025
Processing time: 128 Days and 17.8 Hours
We have innovatively amalgamated membrane blood purification and centrifugal blood cell separation technologies to address the limitations of current artificial liver support (ALS) models, and develop a versatile plasma purification system (VPPS) through centrifugal plasma separation.
To investigate the influence of VPPS on long-term rehospitalization and mortality rates among patients with acute-on-chronic liver failure (ACLF).
This real-world, prospective study recruited inpatients diagnosed with ACLF from the Second Xiangya Hospital of Central South University between October 2021 and March 2024. Patients were categorized into the VPPS and non-VPPS groups based on the distinct ALS models administered to them. Self-administered questionnaires, clinical records, and self-reported data served as the primary methods for data collection. The laboratory results were evaluated at six distinct time points. All patients were subjected to follow-up assessments for > 12 months. Kaplan-Meier survival analyses and Cox proportional hazards models were used to evaluate the risks of hospitalization and mortality during the follow-up period.
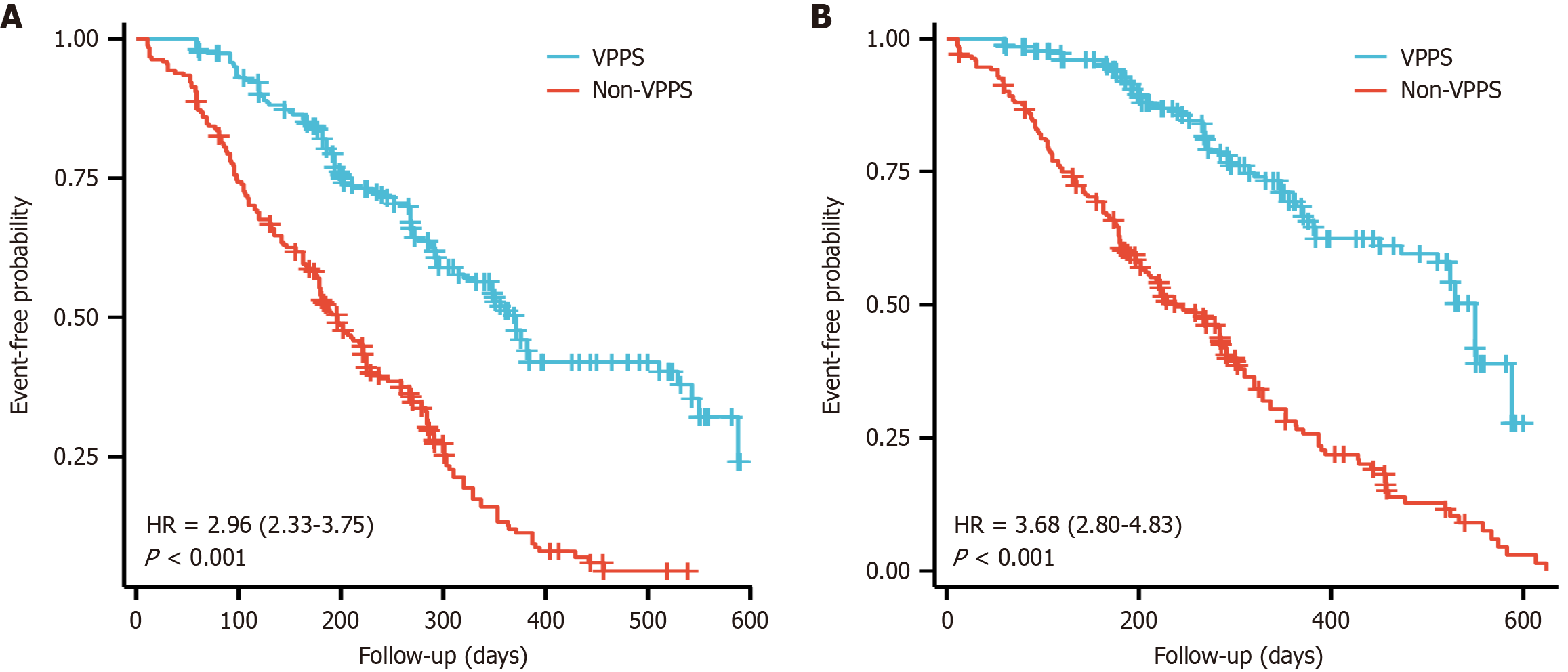
A cohort of 502 patients diagnosed with ACLF was recruited, with 260 assigned to the VPPS group. On comparing baseline characteristics, the VPPS group exhibited a significantly shorter length of stay, higher incidence of spontaneous peritonitis and pulmonary aspergillosis compared to the non-VPPS group (P < 0.05). Age [hazard ratio (HR) = 1.142, 95%CI: 1.01-1.23, P = 0.018), peritonitis (HR = 2.825, 95%CI: 1.07-6.382, P = 0.026), albumin (HR = 0.67, 95%CI: 0.46-0.942, P = 0.023), total bilirubin (HR = 1.26, 95%CI: 1.01-3.25, P = 0.021), international normalized ratio (HR = 1.97, 95%CI: 1.21-2.908, P = 0.014), and VPPS/non-VPPS (HR = 3.24, 95%CI: 2.152-4.76, P < 0.001) were identified as significant independent predictors of mortality in both univariate and multivariate analyses throughout the follow-up period. Kaplan-Meier survival analyses demonstrated significantly higher rehospitalization and mortality rates in the non-VPPS group compared to the VPPS group during follow-up of ≥ 2 years (log-rank test, P < 0.001).
These findings suggest that VPPS is safe and has a positive influence on prognostic outcomes in patients with ACLF.
Core Tip: In this study, we introduced a novel artificial liver support model, termed the Versatile Plasma Purification System (VPPS), aimed at treating patients with acute-on-chronic liver failure (ACLF). We demonstrated that the VPPS significantly enhances liver function, coagulation parameters, and blood ammonia levels in ACLF patients, as evidenced by a comparative analysis of laboratory data obtained prior to and following treatment with both VPPS and non-VPPS modes. Furthermore, our analysis revealed that patients receiving VPPS treatment exhibited reduced rates of readmission and mortality throughout the long-term follow-up period.
- Citation: Dai ZS, Zhang M, Deng YY, Zhou N, Tian Y. Efficacy of a novel artificial liver versatile plasma purification system in patients with acute-on-chronic liver failure. World J Gastroenterol 2025; 31(14): 103892
- URL: https://www.wjgnet.com/1007-9327/full/v31/i14/103892.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i14.103892
Liver failure is a prevalent and severe clinical syndrome characterized by significantly elevated mortality rates[1,2]. Acute-on-chronic liver failure (ACLF) represents a complex clinical syndrome defined by the acute exacerbation of liver function in patients with underlying chronic liver disease, often resulting in elevated mortality associated with both hepatic and extrahepatic organ failure. The short-term mortality rate associated with comprehensive internal medicine management may reach 50%-90%[3-5]. ACLF is influenced by multifactorial triggering factors, exhibits unclear pathogenesis, and is characterized by an absence of targeted therapeutic agents and treatment methods. Consequently, identifying a more scientifically effective approach for the treatment of ACLF presents a significant challenge that necessitates global attention[6].
Artificial liver support (ALS) represents a promising approach for the management of liver failure. The therapeutic mechanism centers on the inherent regenerative capacity of hepatic cells, utilizing an external mechanical apparatus to eliminate toxic substances, supply essential nutrients, and optimize the internal environment[7]. ALS can transiently assume certain functions of the failing liver, thereby facilitating conditions conducive to hepatic regeneration or bridging the interval to liver transplantation[8,9]. Recent clinical investigations have demonstrated that ALS therapy can enhance hepatic and coagulation functions, ameliorate inflammatory responses, and facilitate hepatic repair in liver failure patients[10-12]. Additionally, studies have indicated that patients undergoing ALS therapy exhibit extended survival and reduced hospitalization during follow-up evaluation[13,14]. Currently, however, the efficiency of most ALS technologies is constrained by the limitations of membrane separation coefficients in effectively isolating and removing toxic substances. Additionally, to enhance the clearance efficiency, deep vein catheterization is required to achieve increased blood flow velocity, consequently elevating the risks of catheter-related infection, bleeding, and thrombosis[15]. To address the limitations of current ALS models, we have innovatively amalgamated membrane blood purification and centrifugal blood cell separation technologies to develop a versatile plasma purification system (VPPS) through cen
This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University (No. 2022-53) and was conducted in accordance with the Declaration of Helsinki. We obtained informed consent from all study participants. All methods including diagnosis of ACLF were performed in accordance with European Association for the Study of the Liver (EASL) guidelines and regulations[16].
This real-world, observational, prospective study recruited inpatients diagnosed with ACLF from the Second Xiangya Hospital of Central South University between October 2021 and March 2024. All recruited participants received a definitive diagnosis of ACLF in accordance with EASL guidelines, characterized by the functional failure of one or more of the six major organ systems, accompanied by systemic inflammation potentially induced by acute precipitants[16]. Patients were excluded if they lacked liver function or coagulation function test results, had not received ALS treatment, or if their data were incomplete. Patients were randomly categorized into the VPPS and non-VPPS groups based on the distinct ALS models administered to them.
The following information was recorded at the time of the initial visit: Age, gender, etiology, length of hospital stay, hormone therapy, comorbidities, prognostic score, complete blood count, hepatic and renal function, electrolyte levels, blood ammonia, lactic acid, and coagulation function. Self-administered questionnaires, clinical records, and self-reported data served as the primary methods for data collection. The aforementioned laboratory results were evaluated at six distinct time points: Upon admission, prior to and following ALS treatment, and immediately before discharge. Data collection was conducted by trained research assistants, while the variables were subsequently validated by qualified medical professionals.
In clinical practice, prevalent non-biological ALS models encompass PE, plasma perfusion, hemofiltration (HF), plasmadiafiltration (PDF), PE with HF, double plasma molecular adsorption systems (DPMAS), among other combinatory approaches[7]. VPPS was designed with a specialized purification circuit that integrated centrifugal therapeutic PE (c-TPE) with plasma diafiltration adsorption (PDFA). The treatment protocol comprised two distinct phases: 2 hours of c-TPE followed by 4 hours of PDFA. The three non-VPPS treatment modalities were as follows: PE, PE combined with DPMAS (PE + DPMAS), and PDF. The selection of the non-VPPS treatment modality was guided by established guidelines for non-bioartificial liver support systems in the management of liver failure[16]. All patients were subjected to follow-up assessments for > 12 months. Data regarding the frequency of ACLF rehospitalization during the initial and final years of follow-up, as well as all-cause mortality throughout the follow-up period, were collected by research assistants through telephone consultations with patients and their relatives, outpatient clinic interviews, and reviews of medical records provided by patients. The primary outcomes of this study were hospitalization due to ACLF exacerbation and overall mortality rates.
Data analysis was conducted using SPSS version 21.0 and R software version 3.6. The normality test revealed that the data in this study did not follow a normal distribution. Descriptive statistics for data lacking normal distribution were presented as medians with interquartile ranges (25th and 75th percentiles), while frequencies were reported as counts with corresponding percentages. For categorical variables, intergroup comparisons were performed using either the χ2 test or Fisher's exact test. Continuous variables were compared using one-way analysis of variance, Mann-Whitney U test, or t test.
Kaplan-Meier survival analyses and Cox proportional hazards models were used to evaluate the risks of hospitalization due to ACLF exacerbation and mortality during follow-up. Multivariate Cox regression models were utilized to examine the frequency and risk of rehospitalization throughout follow-up. Potential confounding factors exhibiting P < 0.10 in baseline characteristic analyses were adjusted for in the models. Age and gender were included due to their significant clinical relevance, whereas grouping-related variables were omitted to prevent potential collinearity. A significance threshold of P < 0.05 was established for statistical analyses.
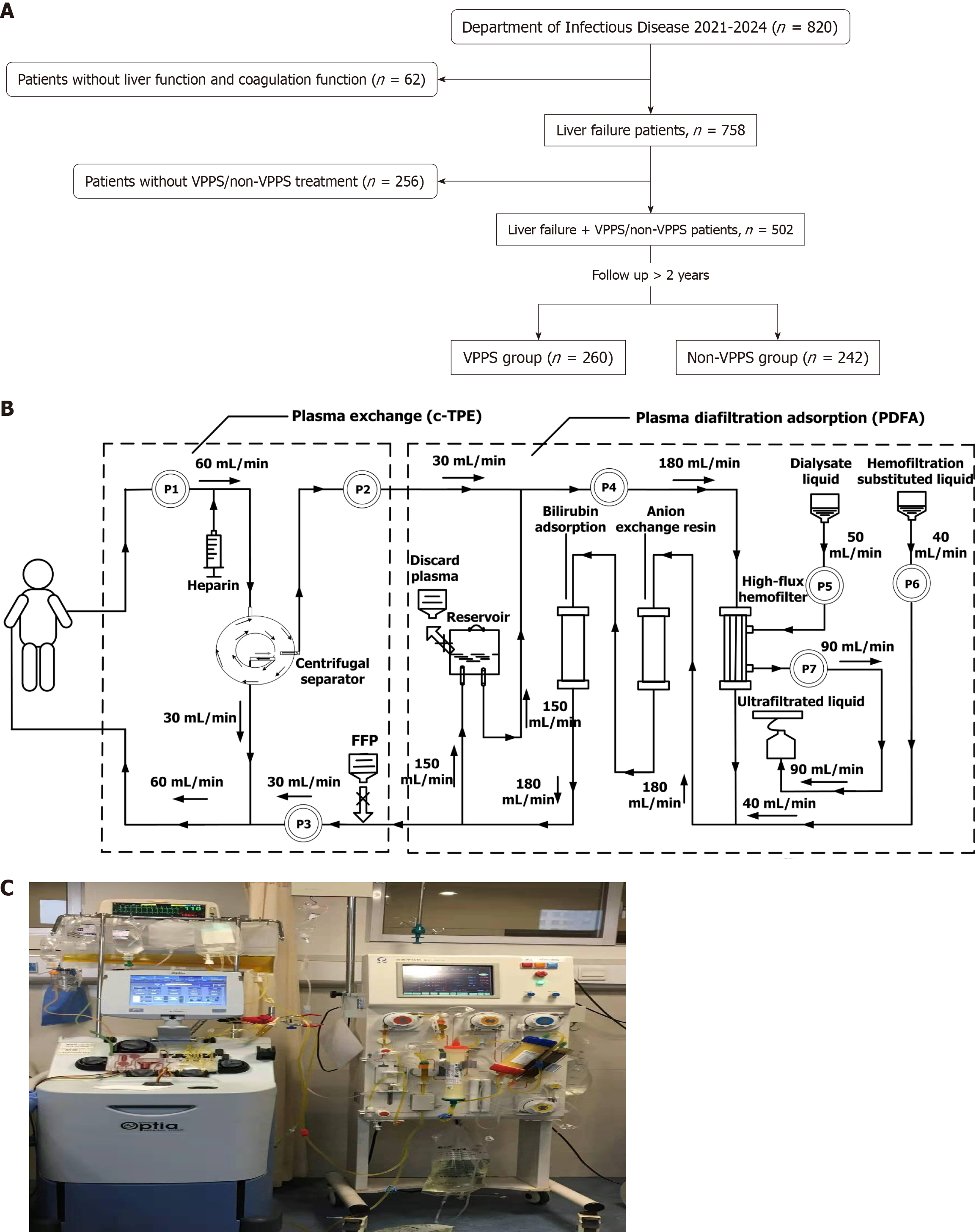
The study flowchart is depicted in Figure 1A. A cohort of 502 ACLF patients was recruited from the Second Xiangya Hospital of Central South University, with 260 patients assigned to the VPPS group. VPPS included a unique purification circuit integrating c-TPE with PDFA. The c-TPE procedure is detailed in Figure 1B. The design of the plasma recycling diafiltration absorption circuit is illustrated in Figure 1C. Detailed operational procedures for the VPPS mode are provided in Supplementary material.
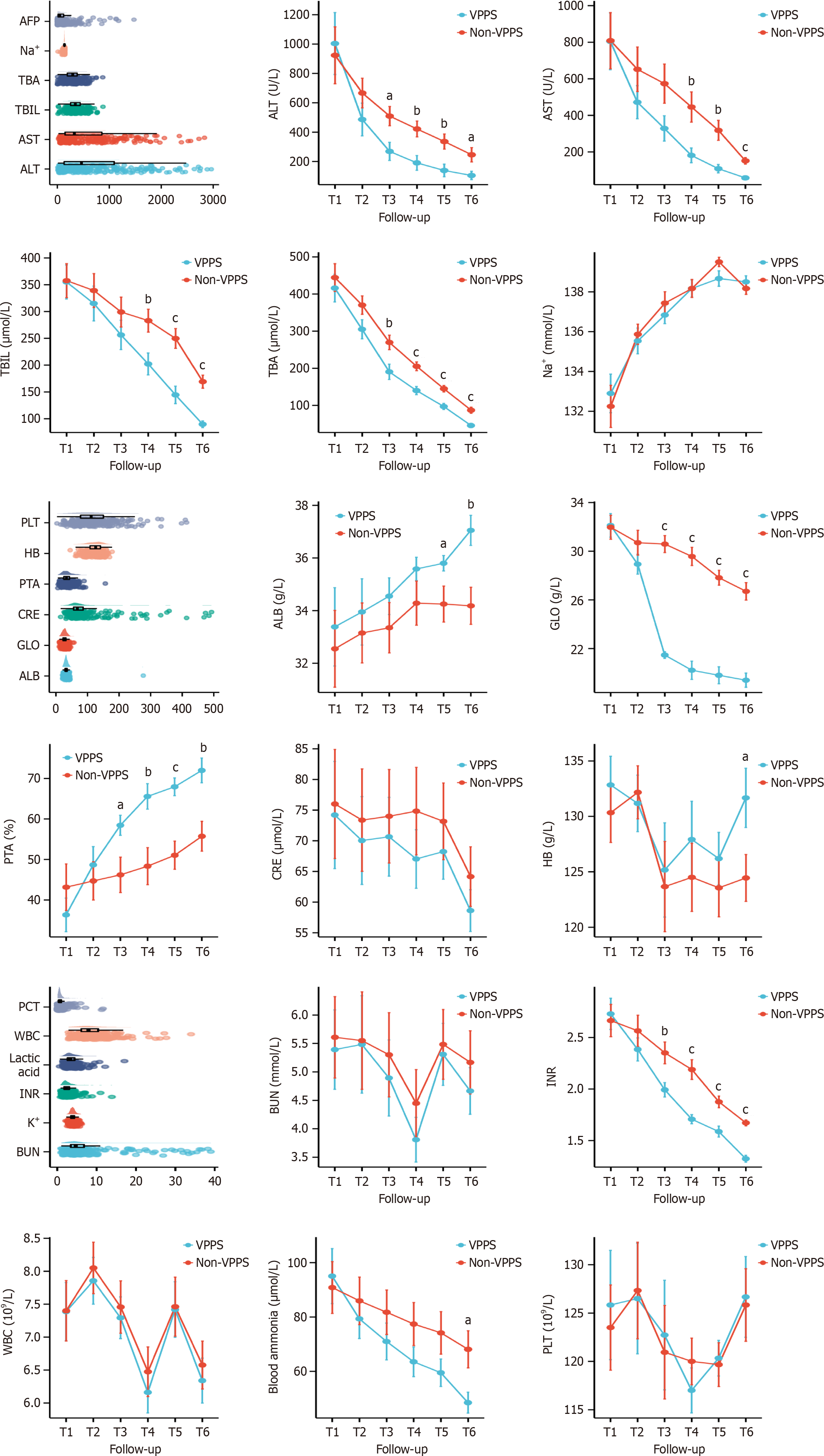
On comparing baseline characteristics, the VPPS group exhibited a significantly shorter length of stay compared to the non-VPPS group (P = 0.001). The incidence of spontaneous peritonitis (P < 0.001) and pulmonary aspergillosis (P = 0.035) was notably higher in the VPPS than in the non-VPPS group. The VPPS group registered higher Chronic Liver Failure (CLIF)-Sequential Organ Failure Assessment and CLIF-OF scores than the non-VPPS group registered (P < 0.05). However, there were no significant differences between the groups in terms of age, gender, comorbid conditions (e.g., hepatic encephalopathy, hepatorenal syndrome, or gastrointestinal hemorrhage), etiology, hormone therapy, and other prognostic scores (Table 1). Baseline laboratory data, including blood routine, hepatic and renal function, electrolytes, blood ammonia, lactic acid, and coagulation function, showed no significant differences between the two groups of ACLF patients at admission. Post-ALS treatment, the VPPS group exhibited significant reductions in alanine transaminase, aspartate transaminase, total bilirubin (TBIL), total bile acid, globulin, international normalized ratio (INR), and blood ammonia levels, alongside notable increases in albumin (ALB), prothrombin activity, and hemoglobin, relative to the non-VPPS group (P < 0.05; Figure 2).
| Demographic characteristics | VPPS (n = 260) | Non-VPPS (n = 242) | P value |
| Age (year) | 49 (38.75, 58) | 47 (37.3, 54.1) | 0.213 |
| Male | 211 (81.15) | 210 (86.77) | 0.155 |
| SaO2, (%) | 98 (97.75, 99) | 98 (97, 98.23) | 0.111 |
| FiO2, (%) | 21 (20, 21) | 21 (20, 22) | 0.224 |
| Etiology | 0.124 | ||
| Hepatitis B | 177 (68.1) | 173 (71.4) | |
| Alcohol | 43 (16.5) | 37 (15.3) | |
| Drug | 33 (12.7) | 27 (11.3) | |
| Autoimmunity | 7 (2.7) | 5 (2) | |
| Length of stay (day) | 23.5 (10, 43) | 28 (20, 40) | 0.001 |
| Hormone therapy | 50 (19.2) | 51 (21.6) | 0.248 |
| Hormone total amount (mg) | 51.2 (10, 60) | 48 (12, 56) | 0.413 |
| Hormone treatment course (day) | 3 (1, 6) | 2 (1, 5) | 0.620 |
| Comorbidities | < 0.001 | ||
| Yes | 247 (95) | 164 (67.6) | |
| No | 13 (5) | 78 (32.4) | |
| Peritonitis | 214 (82.3) | 129 (53.3) | < 0.001 |
| Pulmonary aspergillosis | 13 (5) | 6 (2.4) | 0.035 |
| Hepatic encephalopathy | 148 (56.9) | 129 (53.3) | 0.419 |
| Hepatorenal syndrome | 56 (21.5) | 61 (25.2) | 0.198 |
| Gastrointestinal hemorrhage | 34 (13.1) | 38 (15.7) | 0.090 |
| Prognostic score | |||
| MELD | 25.7 (10, 32) | 23.1 (11, 34.6) | 0.123 |
| MELD-Na | 29.1 (11, 36.4) | 27.3 (9.1, 35) | 0.164 |
| CLIF-SOFA | 9.5 (6, 13) | 7.9 (5, 11) | 0.024 |
| CLIF-OFs | 9.9 (6, 14) | 8.1 (5, 10) | 0.028 |
| CLIF-C ACLF | 45.3 (19, 50.4) | 42.4 (20.2, 51) | 0.198 |
| HBV-SOFA | 5.1 (4, 6) | 4.9 (4, 6) | 0.213 |
| COSSH-ACLF | 7.0 (5, 12) | 6.8 (5, 11) | 0.165 |
| COSSH-ACLF IIs | 8.7 (5.4, 13.6) | 8.1 (5, 12.9) | 0.133 |
Age (HR = 1.142, 95%CI: 1.01-1.23, P = 0.018), peritonitis (HR = 2.825, 95%CI: 1.07-6.382, P = 0.026), ALB (HR = 0.67, 95%CI: 0.46-0.942, P = 0.023), TBIL (HR = 1.26, 95%CI: 1.01-3.25, P = 0.021), INR (HR = 1.97, 95%CI: 1.21-2.908, P = 0.014), and VPPS/non-VPPS (HR = 3.24, 95%CI: 2.152-4.76, P < 0.001) were identified as significant independent predictors of mortality in both univariate and multivariate analyses throughout follow-up (Table 2). Following a 2-year follow-up period, a comparison was made between the VPPS and non-VPPS groups regarding mortality rates and the incidence of rehospitalization in the preceding year. Results revealed that, over the follow-up period, the incidence of rehospitalization and mortality rates were notably lower in the VPPS group relative to the non-VPPS group. Kaplan-Meier survival analyses demonstrated significantly higher rehospitalization and mortality rates in the non-VPPS group compared to the VPPS group over a follow-up duration of ≥ 2 years (log-rank test, P < 0.001; Figure 3).
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| VPPS/non-VPPS | ||||
| VPPS | Reference | Reference | ||
| Non-VPPS | 3.676 (2.799, 4.827) | < 0.001 | 3.24 (2.152, 4.76) | < 0.001 |
| Age | 1.231 (1.03, 1.428) | 0.003 | 1.142 (1.01, 1.23) | 0.018 |
| Sex (male) | 1.225 (1.164, 2.18) | 0.036 | 1.17 (0.886, 2.102) | 0.120 |
| Peritonitis | 3.46 (1.75, 8.52) | 0.012 | 2.825 (1.07, 6.382) | 0.026 |
| ALB | 0.276 (0.128, 0.396) | < 0.001 | 0.67 (0.46, 0.942) | 0.023 |
| TBIL | 1.987 (1.06, 3.963) | < 0.001 | 1.26 (1.01, 3.25) | 0.021 |
| INR | 2.623 (1.05, 3.742) | < 0.001 | 1.97 (1.21, 2.908) | 0.014 |
We introduced a novel ALS model, termed the VPPS, aimed at treating patients with ACLF. We demonstrated that VPPS significantly enhanced liver function, coagulation parameters, and blood ammonia levels in ACLF patients, as demonstrated by a comparative analysis of laboratory data obtained prior to and following treatment with both VPPS and non-VPPS modes. Furthermore, our analysis revealed that patients receiving VPPS treatment exhibited reduced rates of readmission and mortality throughout long-term follow-up. These findings suggest that VPPS is a novel therapeutic option for patients with ACLF, demonstrating superior efficacy in enhancing liver failure indicators and overall prognosis compared to alternative ALS models.
The establishment of a nonbiological artificial liver (NBAL) system is predicated on blood purification techniques, which use external devices to eliminate various harmful substances from the bloodstream through mechanisms including ultrafiltration, adsorption, permeation, diffusion, and filtration[17,18]. In recent years, several studies have concentrated on evaluating the impact of NBAL on the prognosis of patients suffering from liver failure. One study conducted a Bayesian network meta-analysis of randomized controlled trials to assess the impact of ALS systems on survival outcomes among patients with ACLF. The findings indicated that PE significantly enhanced 3-month overall survival compared to standard medical therapy (RR 0.74, 95%CI: 0.6-0.94) and achieved the highest rank on the cumulative ranking curves for overall survival and 3-month transplant-free survival (SUCRA: 87%)[19]. Another study reported that the pooled survival rates at 28 and 90 days were 68.7% (95%CI: 64.5%-72.9%) and 53.4% (95%CI: 45.5%-61.4%), respectively[20]. These analyses suggest that ALS treatment substantially enhances short-term survival rates in patients with ACLF, indicating that such treatment could potentially mitigate elevated mortality rates. Nevertheless, our study revealed that the VPPS had superior advantages in enhancing long-term mortality and readmission rates among patients with ACLF in comparison to alternative ALS systems. Similarly, a multicenter prospective study demonstrated that PE-based non-bioartificial ALS was the sole independent protective factor for prognosis of hepatitis B virus (HBV)-related ACLF (HBV-ACLF) patients at 28 days, 90 days, and 1 year (HR = 0.516, P = 0.001; HR = 0.663, P = 0.010; HR = 0.610, P = 0.051, respectively). Optimizing ALS may enhance prognosis and potentially save lives in patients with HBV-ACLF[21].
The current NBAL system predominantly uses membrane blood separation technology to effectively manage a range of conditions, including liver failure, sepsis, and multiple organ failure. The therapeutic principle involves a peristaltic pump to extricate blood from the body, subsequently using hollow fibers with varying membrane pore sizes to eliminate pathogenic substances and accumulated metabolites in the plasma via methods such as dialysis, filtration, adsorption, and PE[22,23]. Nonetheless, membrane blood separation technology has several drawbacks, including inconsistent impurity separation efficiency and risks of infection and bleeding associated with catheterization. We have addressed the limitations of prior ALS technologies by integrating membrane blood purification technology with centrifugal blood cell separation techniques. Our results corroborated that the VPPS model yielded a significantly enhanced therapeutic effect on improving liver function, coagulation function, and blood ammonia levels, with its long-term survival outcomes appearing superior to those of traditional modalities. Agarwal et al[24] designed a novel liver dialysis device, DIALIVE, intended to facilitate the exchange of dysfunctional ALB and eliminate damage- and pathogen-associated molecular patterns. Patients with alcohol-related ACLF received treatment with DIALIVE for up to 5 days, with endpoint assessments conducted at day 10. The study observed a significant reduction in the severity of endotoxemia and an improvement in ALB function within the DIALIVE group, which correlated with a notable decrease in CLIF-C organ failure scores (P = 0.018) and CLIF-C ACLF scores (P = 0.042) at day 10. CytoSorb is an in vitro blood purification device designed to adsorb cytokines and regulate systemic inflammation, primarily used for treatment of septic shock and liver failure. Recently, a study compared the efficacy of CytoSorb with that of the Molecular Adsorbent Recirculating System (MARS) in treating patients with liver failure. The results indicate that CytoSorb may confer potential benefits in enhancing liver function among patients with liver failure relative to MARS; however, its impact on long-term survival outcomes remains uncertain[25].
Age, peritonitis, ALB, TBIL, INR, and the VPPS/non-VPPS status were identified as independent predictors within the ACLF risk score. Prior studies have identified several of these variables as significant risk factors associated with severe outcomes in ACLF. A prospective observational study enrolled patients with ACLF predominantly attributable to alcoholic hepatitis. The study indicated that the coupled plasma-filtration and adsorption (CPFA) system demonstrated favorable hemodynamic tolerance. Following a single CPFA treatment, TBIL, indirect bilirubin, and bile acids exhibited significant reductions without notable rebound effects[26]. A Japanese study demonstrated that high-flow continuous hemodiafiltration enhanced levels of consciousness, with the recovery rate significantly increasing in tandem with elevated dialysate flow and filtrate rates (QD = 500 mL/minutes, P < 0.001)[27]. A recent study sought to evaluate the safety and efficacy of the DPMAS system in conjunction with sequential low-volume PE (LPE) treatment for inter
This study had several limitations. Firstly, the study cohort was not population-based, as patients were exclusively recruited from a tertiary hospital in China. Consequently, the results may not be generalized to the broader population of patients with ACLF. Nevertheless, we assert that our findings possess significant clinical implications and provide an objective evaluation of the efficacy of the VPPS, thereby reflecting the current clinical characteristics and prognosis of patients with ACLF. Secondly, the limited number of deaths during the follow-up period constrained our capacity to identify potentially significant associations. Confounding factors arising from unmeasured variables, including comorbidities and medication during follow-up, may also impact clinical outcomes. Long-term follow-up data from patients in other settings will be collected and analyzed in future studies.
These findings suggest that VPPS is safe and positively influences prognostic outcomes in patients with ACLF. Larger, adequately powered clinical studies are necessary to provide further confirmation of its safety and efficacy.
We would like to thank all participants for collecting the data of this study.
| 1. | Yuan X, Wu J, Sun Z, Cen J, Shu Y, Wang C, Li H, Lin D, Zhang K, Wu B, Dhawan A, Zhang L, Hui L. Preclinical efficacy and safety of encapsulated proliferating human hepatocyte organoids in treating liver failure. Cell Stem Cell. 2024;31:484-498.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 2. | Fischer P, Stefanescu H, Hategan R, Procopet B, Ionescu D. Bacterial infection-related acute-on-chronic liver failure: The standpoint matters! J Hepatol. 2021;75:1009-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382:2137-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 429] [Article Influence: 85.8] [Reference Citation Analysis (2)] |
| 4. | Moreau R, Gao B, Papp M, Bañares R, Kamath PS. Acute-on-chronic liver failure: A distinct clinical syndrome. J Hepatol. 2021;75 Suppl 1:S27-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (1)] |
| 5. | Shi R, Hui X, Tong T, Li J, Zhang L, Yang K. Non-bioartificial artificial liver support system in acute liver failure: A comprehensive systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2025;49:102527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 6. | Artru F, Trovato F, Morrison M, Bernal W, McPhail M. Liver transplantation for acute-on-chronic liver failure. Lancet Gastroenterol Hepatol. 2024;9:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 7. | Saliba F, Bañares R, Larsen FS, Wilmer A, Parés A, Mitzner S, Stange J, Fuhrmann V, Gilg S, Hassanein T, Samuel D, Torner J, Jaber S. Artificial liver support in patients with liver failure: a modified DELPHI consensus of international experts. Intensive Care Med. 2022;48:1352-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Maiwall R, Kulkarni AV, Arab JP, Piano S. Acute liver failure. Lancet. 2024;404:789-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Reference Citation Analysis (0)] |
| 9. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 10. | Li J, Liang X, You S, Feng T, Zhou X, Zhu B, Luo J, Xin J, Jiang J, Shi D, Lu Y, Ren K, Wu T, Yang L, Li J, Li T, Cai Q, Sun S, Guo B, Zhou X, Chen J, He L, Li P, Yang H, Hu W, An Z, Jin X, Tian J, Wang B, Chen X, Xin S, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J Hepatol. 2021;75:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Maiwall R, Bajpai M, Singh A, Agarwal T, Kumar G, Bharadwaj A, Nautiyal N, Tevethia H, Jagdish RK, Vijayaraghavan R, Choudhury A, Mathur RP, Hidam A, Pati NT, Sharma MK, Kumar A, Sarin SK. Standard-Volume Plasma Exchange Improves Outcomes in Patients With Acute Liver Failure: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2022;20:e831-e854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 12. | Matchett KP, Wilson-Kanamori JR, Portman JR, Kapourani CA, Fercoq F, May S, Zajdel E, Beltran M, Sutherland EF, Mackey JBG, Brice M, Wilson GC, Wallace SJ, Kitto L, Younger NT, Dobie R, Mole DJ, Oniscu GC, Wigmore SJ, Ramachandran P, Vallejos CA, Carragher NO, Saeidinejad MM, Quaglia A, Jalan R, Simpson KJ, Kendall TJ, Rule JA, Lee WM, Hoare M, Weston CJ, Marioni JC, Teichmann SA, Bird TG, Carlin LM, Henderson NC. Multimodal decoding of human liver regeneration. Nature. 2024;630:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 13. | Yang Z, Zhang Z, Cheng Q, Chen G, Li W, Ma K, Guo W, Luo X, Chen T, Ning Q. Plasma perfusion combined with plasma exchange in chronic hepatitis B-related acute-on-chronic liver failure patients. Hepatol Int. 2020;14:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Xu W, Zhu S, Yang L, Li Z, Wu L, Zhang Y, Chen J, Deng Z, Luo Q, Peng L. Safety and efficacy of double plasma molecular adsorption system with sequential low-volume plasma exchange in intermediate-stage hepatitis B virus-related acute-on-chronic liver failure. J Med Virol. 2023;95:e28650. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Wang XH, Peng BB, Zhang L, Zhao J, Zhang L, Ren H, Hu P, Li H, Zhong S. Mixed mode of artificial liver support in patients with acute-on-chronic liver failure: a retrospective cohort study. Hepatol Int. 2023;17:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J Hepatol. 2023;79:461-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 139] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 17. | Br VK, Sarin SK. Acute-on-chronic liver failure: Terminology, mechanisms and management. Clin Mol Hepatol. 2023;29:670-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 18. | Brown RS Jr, Fisher RA, Subramanian RM, Griesemer A, Fernandes M, Thatcher WH, Stiede K, Curtis M. Artificial Liver Support Systems in Acute Liver Failure and Acute-on-Chronic Liver Failure: Systematic Review and Meta-Analysis. Crit Care Explor. 2025;7:e1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Ocskay K, Kanjo A, Gede N, Szakács Z, Pár G, Erőss B, Stange J, Mitzner S, Hegyi P, Molnár Z. Uncertainty in the impact of liver support systems in acute-on-chronic liver failure: a systematic review and network meta-analysis. Ann Intensive Care. 2021;11:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Li G, Zhang P, Zhu Y. Artificial liver support systems for hepatitis B virus-associated acute-on-chronic liver failure: A meta-analysis of the clinical literature. J Viral Hepat. 2023;30:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Chen YY, Li H, Xu BY, Zheng X, Li BL, Wang XB, Huang Y, Gao YH, Qian ZP, Liu F, Lu XB, Shang J, Li H, Wang SY, Zhang YH, Meng ZJ; Chinese Chronic Liver Failure (CLIF) Consortium. Plasma Exchange-Based Non-bioartificial Liver Support System Improves the Short-Term Outcomes of Patients With Hepatitis B Virus-Associated Acute-on-Chronic Liver Failure: A Multicenter Prospective Cohort Study. Front Med (Lausanne). 2021;8:779744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Ma Y, Xu Y, Du L, Bai L, Tang H. Outcome of patients with different stages of acute-on-chronic liver failure treated with artificial liver support system. Front Med (Lausanne). 2024;11:1381386. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Feng L, Wang Y, Fu Y, Yimamu A, Guo Z, Zhou C, Li S, Zhang L, Qin J, Liu S, Xu X, Jiang Z, Cai S, Zhang J, Li Y, Peng Q, Yi X, He G, Li T, Gao Y. A simple and efficient strategy for cell-based and cell-free-based therapies in acute liver failure: hUCMSCs bioartificial liver. Bioeng Transl Med. 2023;8:e10552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Agarwal B, Cañizares RB, Saliba F, Ballester MP, Tomescu DR, Martin D, Stadlbauer V, Wright G, Sheikh M, Morgan C, Alzola C, Lavin P, Green D, Kumar R, Sacleux SC, Schilcher G, Koball S, Tudor A, Minten J, Domenech G, Aragones JJ, Oettl K, Paar M, Waterstradt K, Bode-Boger SM, Ibáñez-Samaniego L, Gander A, Ramos C, Chivu A, Stange J, Lamprecht G, Sanchez M, Mookerjee RP, Davenport A, Davies N, Pavesi M, Andreola F, Albillos A, Cordingley J, Schmidt H, Carbonell-Asins JA, Arroyo V, Fernandez J, Mitzner S, Jalan R. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on- chronic liver failure. J Hepatol. 2023;79:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 25. | Popescu M, David C, Marcu A, Olita MR, Mihaila M, Tomescu D. Artificial Liver Support with CytoSorb and MARS in Liver Failure: A Retrospective Propensity Matched Analysis. J Clin Med. 2023;12:2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 26. | Donati G, Angeletti A, Gasperoni L, Piscaglia F, Croci Chiocchini AL, Scrivo A, Natali T, Ullo I, Guglielmo C, Simoni P, Mancini R, Bolondi L, La Manna G. Detoxification of bilirubin and bile acids with intermittent coupled plasmafiltration and adsorption in liver failure (HERCOLE study). J Nephrol. 2021;34:77-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | Fujiwara K, Abe R, Yasui S, Yokosuka O, Kato N, Oda S. High recovery rate of consciousness by high-volume filtrate hemodiafiltration for fulminant hepatitis. Hepatol Res. 2019;49:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |