Published online Mar 21, 2025. doi: 10.3748/wjg.v31.i11.103412
Revised: January 10, 2025
Accepted: February 8, 2025
Published online: March 21, 2025
Processing time: 107 Days and 4.5 Hours
The upregulation of serpin family B member 5 (SERPINB5) has been linked to the progression of rectal cancer. However, the specific roles and underlying me
To investigate the roles and mechanisms of SERPINB5 in rectal cancer.
SERPINB5 protein level in rectal cancer tissues and cell lines was measured through western blot analysis. SW480 cells were transfected with pcDNA-SERPINB5 or short-hairpin RNA targeting SERPINB5 (sh-SERPINB5). Cell proliferation, invasion, and apoptosis were then evaluated. The interaction between SERPINB5 and heat shock protein 90 alpha class A member 1 (HSP90AA1) was confirmed through a co-immunoprecipitation assay. Subsequently, pcDNA-HSP90AA1 or sh-HSP90AA1 was transfected into SW480 cells, and cell pro
SERPINB5 was prominently upregulated in rectal cancer tissues and cells. SERPINB5 overexpression increased SW480 cell proliferation and invasion while reducing apoptosis. In contrast, SERPINB5 knockdown had the opposite effects. Moreover, SERPINB5 could interact with HSP90AA1 and promote HSP90AA1 expression in SW480 cells. HSP90AA1 overexpression facilitated SW480 cell proliferation and invasion and restrained apoptosis. By contrast, HSP90AA1 knockdown suppressed cell progression. The upregulation of HSP90AA1 reversed the SERPINB5 silencing-mediated inhibition of SW480 cell progression. Additionally, SERPINB5 knockdown retarded the growth of rectal cancer tumors in vivo.
SERPINB5 knockdown inhibited rectal cancer cell proliferation and invasion and retarded xenograft tumor growth by inhibiting HSP90AA1 expression.
Core Tip: This study suggested that serpin family B member 5 (SERPINB5) was significantly upregulated in rectal cancer tissues and cell lines. SERPINB5 overexpression increased SW480 cell proliferation and invasion while reducing apoptosis. By contrast, SERPINB5 knockdown had the opposite effects. Moreover, SERPINB5 knockdown retarded the growth of rectal cancer tumors in vivo. Mechanistically, SERPINB5 interacted with heat shock protein 90 alpha family class A member 1 and promoted heat shock protein 90 alpha family class A member 1 expression in SW480 cells.
- Citation: Meng ZS, Hu JT, Wu H, Li BK. Inhibition of the SERPINB5/HSP90AA1 axis restrains the proliferation and invasion of rectal cancer. World J Gastroenterol 2025; 31(11): 103412
- URL: https://www.wjgnet.com/1007-9327/full/v31/i11/103412.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i11.103412
Rectal cancer is the fourth leading cause of cancer-associated deaths worldwide[1], and the age of the patient population with this malignancy is gradually skewing younger[2]. Rectal cancer has unremarkable early symptoms in the po
Serpin family B member 5 (SERPINB5), also known as maspin, is a mammary serine protease inhibitor and was found to be aberrantly expressed in numerous cancers[6]. SERPINB5 protein was first reported to be downregulated in multiple breast cancer cell lines[7]. SERPINB5 was classified as a tumor suppressor and promising diagnostic marker in breast and prostate cancers[6,8]. However, SERPINB5 also acts as an oncogene in several cancer types. The upregulated nuclear and cytoplasmic expression of SERPINB5 was found in non-small cell lung cancer, serving as a promising independent prognostic factor in non-small cell lung cancer[9]. Moreover, the cancer cell-derived matrisome protein SERPINB5 is substantially overexpressed in pancreatic ductal adenocarcinoma. SERPINB5 facilitates metastasis and tumorigenesis and is associated with poor patient prognosis[10]. Notably, SERPINB5 has been recently identified as a dramatically upre
Heat shock protein 90 alpha family class A member 1 (HSP90AA1, also known as HSP90 or HSP90A) is a member of the HSP family, which is efficiently expressed under the stimulation of infection, trauma, and tumors[12]. It mainly participates in the maintenance of proteostasis and activates numerous oncogenic client proteins in cancer cells, thereby stimulating cancer cell growth and invasiveness[13-15]. A growing body of evidence demonstrates that HSP90AA1 participates in facilitating proliferation, invasion, and metastasis in several cancer cell[15,16]. A recent study has provided evidence that HSP90AA1 mRNA is remarkably elevated in colorectal cancer tissues[17]. However, the effects of HSP90AA1 on the development of rectal cancer have been rarely explored. This study confirmed that SERPINB5 is prominently upregulated in rectal cancer tissues and cell lines and investigated the roles and mechanisms of the SERPINB5/HSP90AA1 axis in rectal cancer progression, aiming to find a promising direction for rectal cancer treatment.
We recruited patients with rectal cancer (n = 27) at the Fourth Hospital of Hebei Medical University. Patients who underwent chemotherapy, radiotherapy, and other anticancer therapies were excluded. Clinical tumor tissues and matched adjacent tissues were harvested during surgical resection. All patients provide written informed consent. This study was approved by the Medical Ethics Committee of the Fourth Hospital of Hebei Medical University.
Rectal cancer cell lines (HR8348, SW480, SW837, and SW1463) were obtained from the American Type Culture Collection (Manassas, VA, United States). All cells were maintained in Dulbecco’s modified eagle medium (DMEM) containing 10% fetal bovine serum and 1% antibiotics at 37 °C in 5% CO2. The overexpression plasmids pcDNA-SERPINB5 and pcDNA-HSP90AA1, empty vector, short hairpin RNA targeting SERPINB5 (sh-SERPINB5), sh-HSP90AA1, and sh-NC originated from RiboBio (Guangzhou, China). Cells were transfected with pcDNA or siRNA upon reaching 60% confluence by using Lipofectamine™ 3000 (Invitrogen, United States).
Cancer cells were lysed in cold radio immunoprecipitation assay lysis solution to isolate proteins. A total of 20 μg of proteins was separated through sodium-dodecyl sulfate gel electrophoresis and transferred to polyvinylidene fluoride membranes. After being blocked with 5% nonfat milk, the membranes were treated with primary antibodies overnight at 4 °C and later incubated with the secondary antibody for 2 hours. Protein signals were visualized by using enhanced chemiluminescence solution and analyzed with ImageJ software. The relative protein expression level was normalized on the basis of glyceraldehyde-3-phosphate dehydrogenase. The primary antibodies used were SERPINB5 (1:1000, ab182785, Abcam), HSP90AA1 (1:1000, ab13492, Abcam), and glyceraldehyde-3-phosphate dehydrogenase (1:2500, ab9485, Abcam).
Transfected SW480 cells (2 × 103 cells/well) were inoculated into a 96-well plate at 37 °C. Subsequently, they were added with Cell Counting Kit-8 (Dojindo, Japan) solution (10 μL) at 0, 24, 48, and 72 hours of incubation and incubated for 2 hours. Absorbance was examined at 450 nm by using a microplate reader (Varioskan LUX, Thermo Fisher).
A Transwell chamber (8 μm, Millipore Corporation, United States) precoated with 50 μL of Matrigel was used. Transfected SW480 cells and serum-free DMEM were added to the upper chamber (2 × 105/mL, 100 μL). Complete DMEM (500 μL) was introduced into the lower chamber. After 24 hours, invaded cells were stained with 0.1% crystal violet for 30 minutes. Cell numbers were counted by using a light microscope (Olympus, Tokyo, Japan).
Annexin V-fluorescein isothiocyanate (5 μL) and propidium iodide (5 μL) (Invitrogen, United States) were added to the SW480 cell suspension for 15 minutes under dark conditions. Flow cytometry (BD Biosciences, NJ, United States) was used for apoptosis evaluation.
The potential interacting proteins of SERPINB5 were explored using the STRING database (https://cn.string-db.org/). First, we entered the protein name (SERPINB5) and selected the organism as “Homo sapiens” in the “Search” item, and then click “SEARCH”. After selecting the correct protein information, we clicked “Continue,” and all predicted proteins that have potential interacting relationship with SERPINB5 were shown in the image.
Intracellular proteins were incubated with the anti-SERPINB5 antibody or anti-HSP90AA1 antibody at 4 °C overnight, then incubated with protein A/G agarose beads overnight at 4 °C. Immunoprecipitation products were harvested and analyzed by using western blot analysis.
BALB/c nude mice (4-5 weeks old, 16-22 g) were purchased from the Experimental Animal Center of Hebei Medical University. Mice were maintained in standard conditions (22 ± 3 °C, 40%-60% humidity, and 12 hours light/dark cycle) with free access to food and water. Animal experiments were approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 20250158). SW480 cells were transfected with sh-SERPINB5 or sh-NC and subcutaneously injected into the left flanks of mice (n = 8 per group). Tumor volume was calculated every 7 days regularly. Next, the mice were euthanized 28 days after implantation, and tumors were completely removed and imaged. Tumor weight was recorded. Finally, tumor tissues were subjected to western blot and immunohistochemistry (IHC) analyses.
Paraffin-embedded tumor tissue sections (4 μm) were meticulously prepared and subjected to a series of treatments. First, they were cleared of paraffin in xylene, rehydrated, and microwaved by using a sodium citrate solution. After being blocked, the sections were treated with the Ki-67 antibody (ab16667, Abcam) and incubated overnight at 4 °C. Subsequently, a secondary antibody was introduced to the sections, which were then incubated for 1 hour. The sections were subsequently stained by using a diaminobenzidine kit and examined under a light microscope.
Data presented as mean ± SEM were subjected to analysis by using SPSS 22.0. Student’s t-test was employed for statistical comparisons between two groups, whereas one-way analysis of variance was utilized for assessing differences among several groups. P < 0.05 was defined as statistically significant.
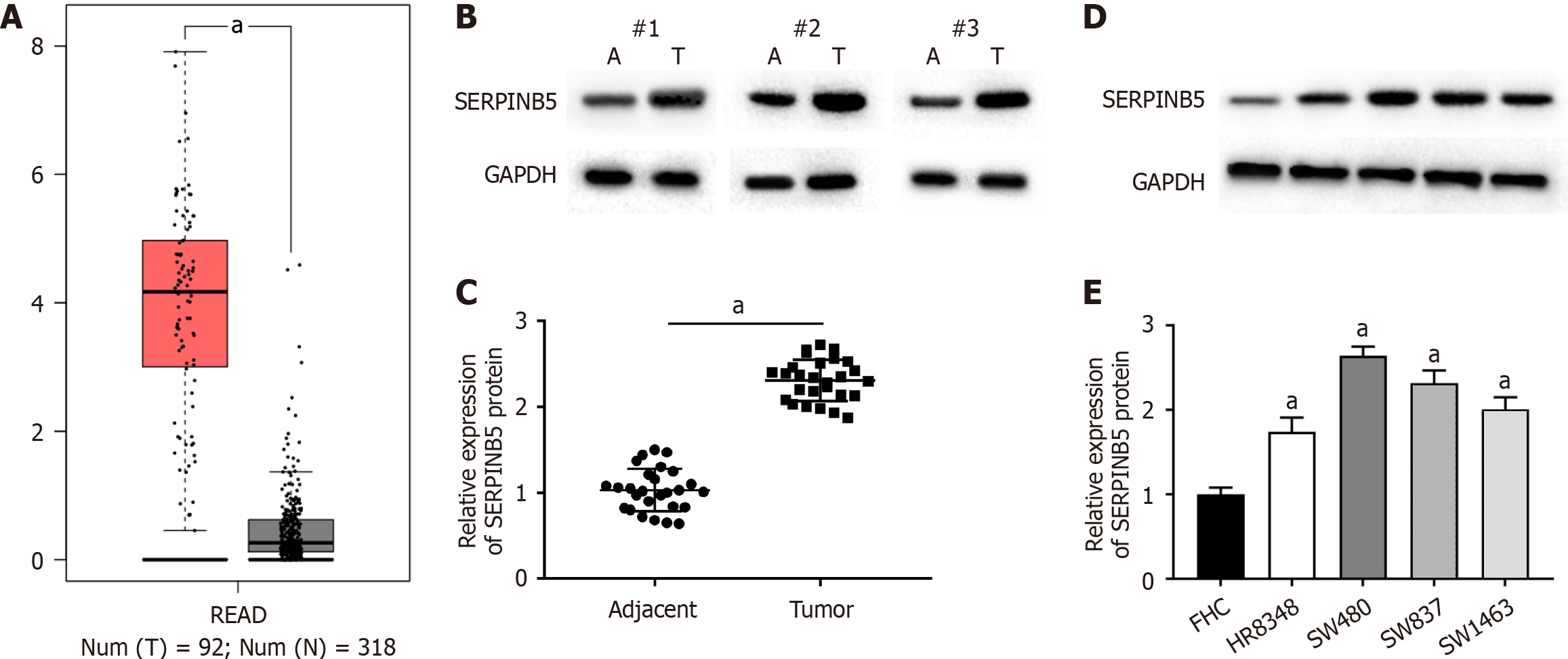
The Gene Expression Profiling Interactive Analysis (GEPIA) online database indicated that SERPINB5 was upregulated in rectal cancer tissues (Figure 1A). Subsequently, western blot analysis confirmed that SERPINB5 was upregulated in rectal cancer tissues relative to matched adjacent tissues (Figure 1B and C). Moreover, SERPINB5 was significantly upregulated in rectal cancer cell lines relative to the normal colonic mucosa cell line FHC (Figure 1D and E).
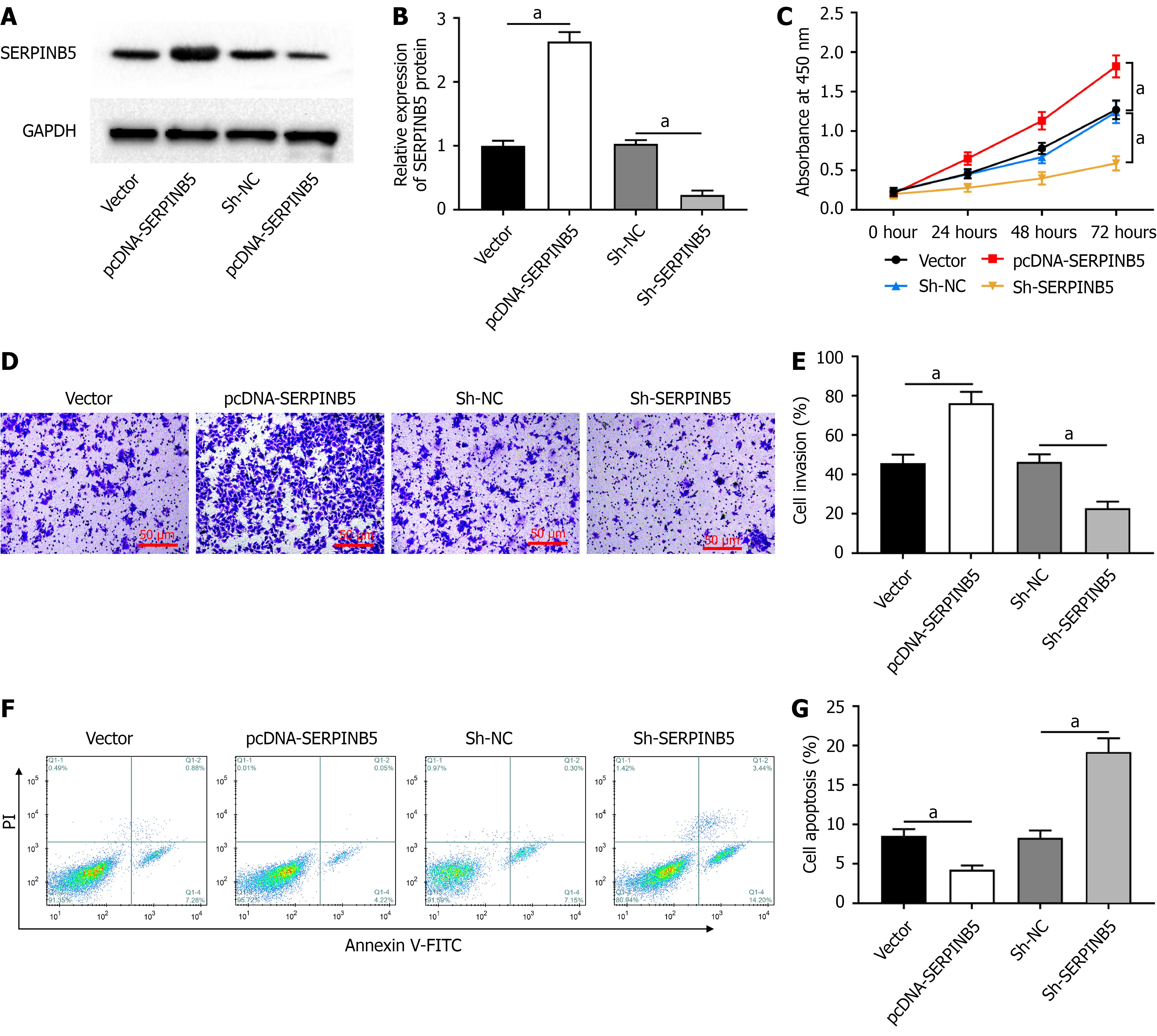
SW480 cells were transfected with pcDNA-SERPINB5 or sh-SERPINB5. pcDNA-SERPINB5 transfection promoted SERPINB5 protein expression in SW480 cells (Figure 2A and B). In contrast, sh-SERPINB5 transfection inhibited SERPINB5 protein expression. Moreover, SERPINB5 overexpression promoted SW480 cell proliferation (Figure 2C) and invasion (Figure 2D and E). However, SERPINB5 knockdown inhibited cell proliferation and invasion. Additionally, in SW480 cells, SERPINB5 overexpression attenuated apoptosis, whereas SERPINB5 knockdown enhanced apoptosis (Figure 2F and G).
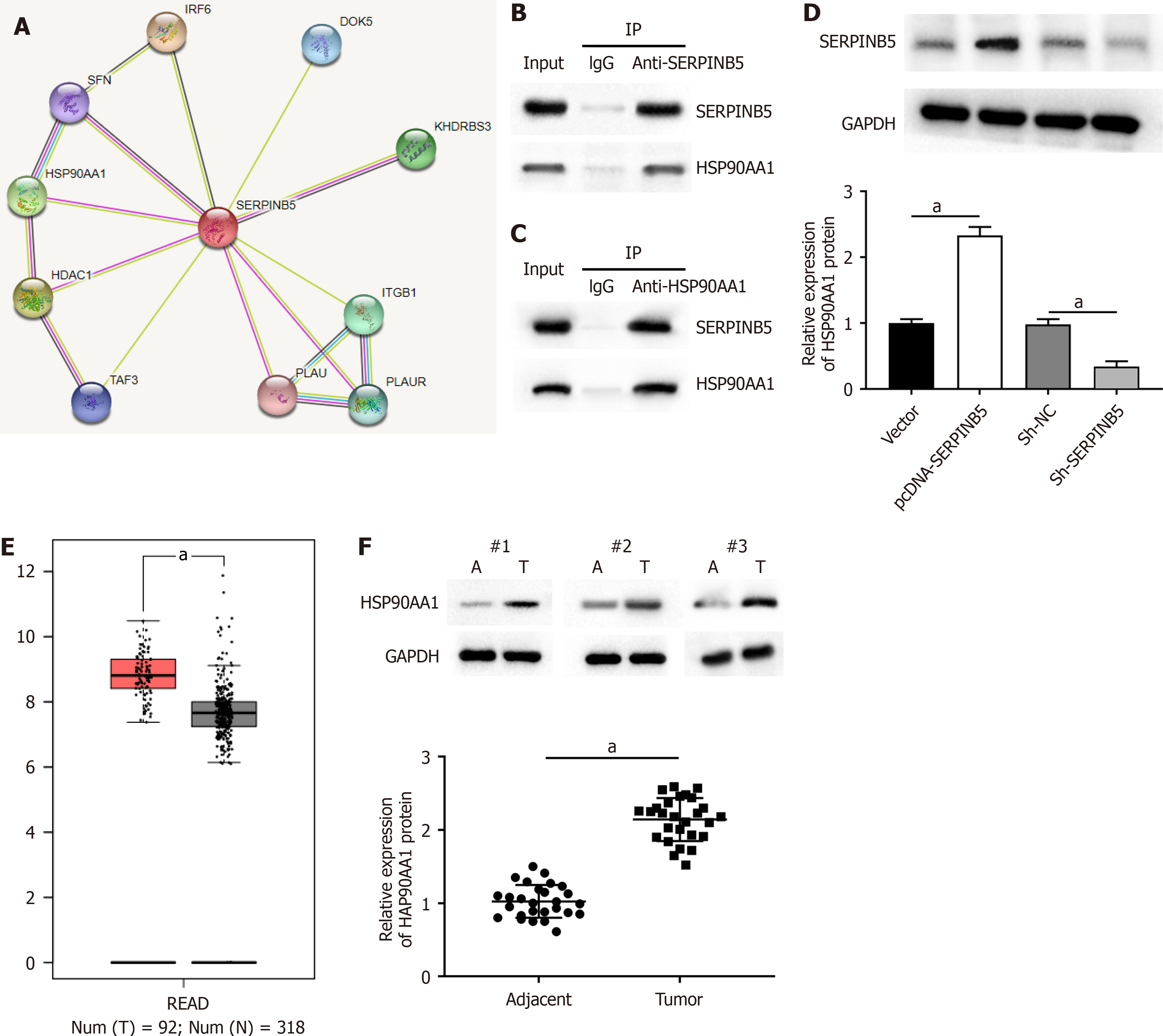
The STRING database showed the existence of a potential interacting relationship between SERPINB5 and HSP90AA1 (Figure 3A). Next, co-immunoprecipitation assay demonstrated that SERPINB5 could interact with HSP90AA1 in SW480 cells (Figure 3B and C). Furthermore, SERPINB5 overexpression markedly augmented HSP90AA1 levels in SW480 cells (Figure 3D). Conversely, SERPINB5 knockdown reduced HSP90AA1 expression (Figure 3D). These results indicated that SERPINB5 interacted with HSP90AA1 and positively regulated HSP90AA1 expression in SW480 cells. Additionally, the GEPIA online database showed that HSP90AA1 was dramatically upregulated in rectal cancer (Figure 3E). Consistent with the results from GEPIA database, we confirmed that HSP90AA1 was upregulated in rectal cancer tissues (Figure 3F).
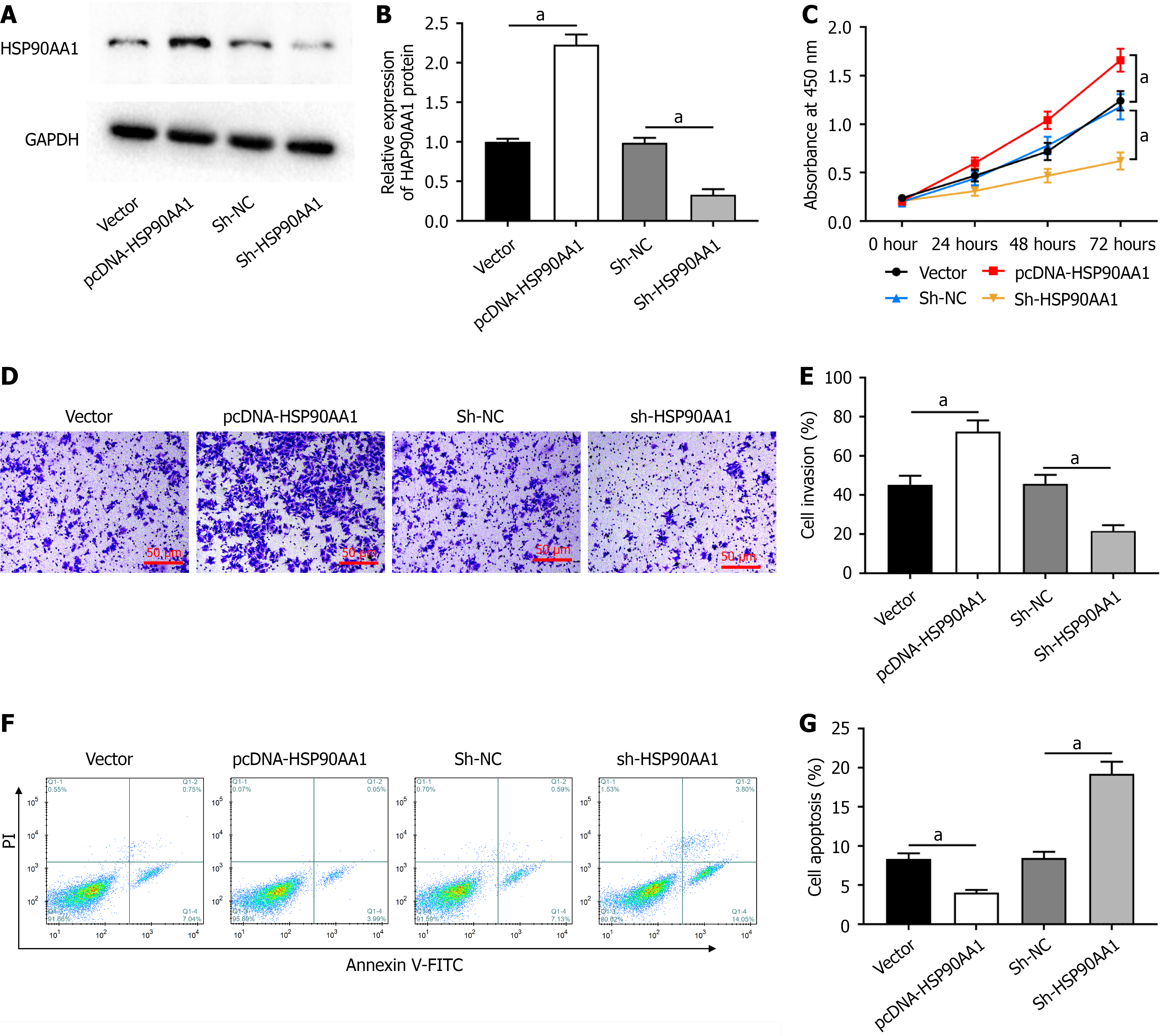
SW480 cells were transfected with pcDNA-HSP90AA1 or sh-HSP90AA1. The results of western blot analysis showed that in SW480 cells, pcDNA-HSP90AA1 transfection promoted HSP90AA1 protein expression (Figure 4A and B). In contrast, sh-HSP90AA1 transfection inhibited the protein expression of HSP90AA1 (Figure 4A and B). Moreover, HSP90AA1 overexpression substantially inhibited proliferation (Figure 4C) and invasion (Figure 4D and E) in SW480 cells. HSP90AA1 knockdown showed the opposite results. In addition, HSP90AA1 overexpression remarkably inhibited apoptosis in SW480 cells, whereas HSP90AA1 knockdown promoted apoptosis (Figure 4F and G).
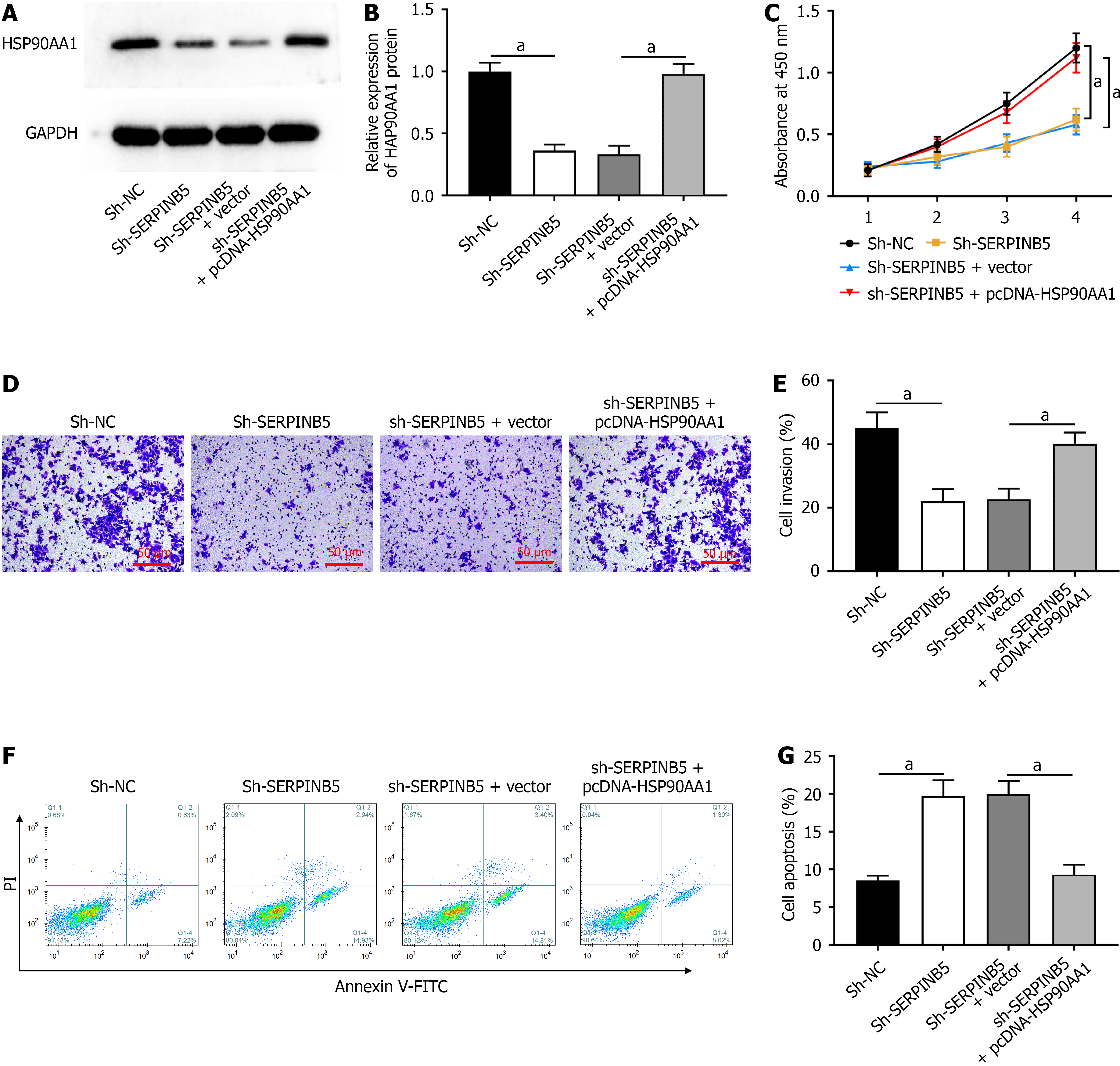
Sh-SERPINB5 and pcDNA-HSP90AA1 were transfected into SW480 cells to explore whether SERPINB5 plays roles in rectal cancer progression by regulating HSP90AA1 expression. Sh-SERPINB5 transfection decreased HSP90AA1 expression, whereas pcDNA-HSP90AA1 transfection inhibited HSP90AA1 expression (Figure 5A and B). Subsequently, SERPINB5 knockdown substantially restrained SW480 cell proliferation (Figure 5C) and invasion (Figure 5D and E). In contrast, HSP90AA1 overexpression reversed these effects. Moreover, SERPINB5 knockdown facilitated SW480 cell apoptosis, which was abolished by HSP90AA1 overexpression (Figure 5F and G). Collectively, our results revealed that SERPINB5 knockdown inhibited rectal cancer progression by inhibiting HSP90AA1 expression.
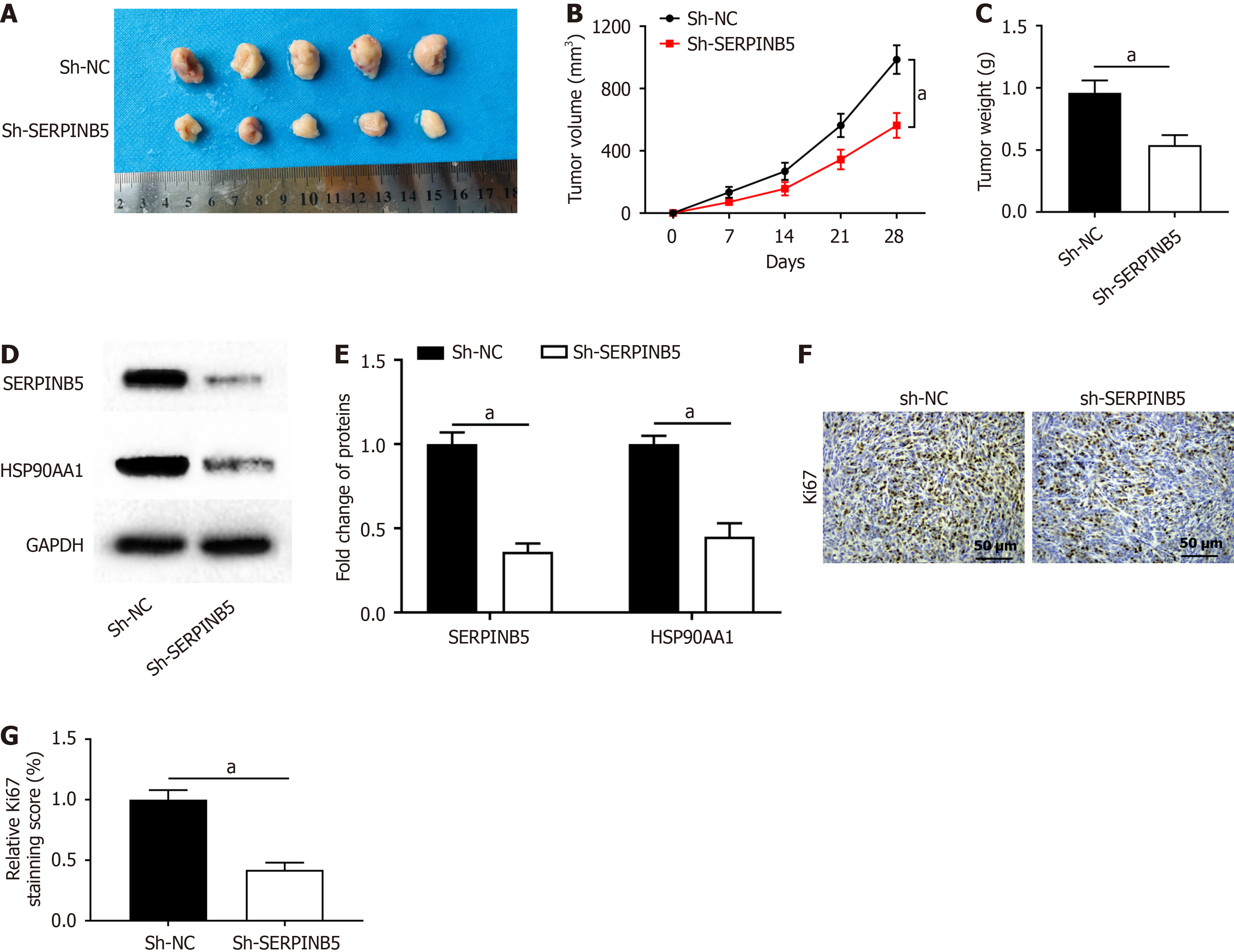
SW837 cells transfected with sh-SERPINB5 or sh-NC were injected into mice to test whether SERPINB5 functions in a xenograft tumor mouse model. We found that SERPINB5 knockdown decreased tumor volume and weight (Figure 6A-C). Moreover, SERPINB5 knockdown suppressed the levels of SERPINB5 and HSP90AA1 protein in tumors (Figure 6D and E). IHC staining results illustrated that SERPINB5 knockdown markedly reduced the levels of the proliferation marker Ki67 (Figure 6F and G). These in vivo data revealed that SERPINB5 knockdown retarded tumor growth in rectal cancer.
Rectal cancer is the main hypotype of colorectal cancer[18]. It is a common malignant tumor that is characterized by low survival rates and poor prognosis. Although considerable advancements in rectal cancer treatment have been made, the prognosis of patients with rectal cancer remains unsatisfactory[5]. Multiple genetic alterations and biological process alterations occur in the development of rectal cancer. Therefore, the in-depth investigation of the mechanisms associated with rectal cancer is urgently needed.
Previous studies have mainly focused on the tumor inhibition effect of SERPINB5. However, SERPINB5 has also been found to serve as an oncogene in several cancers. SERPINB5 has been illustrated to be upregulated in gallbladder cancer[19], thyroid cancer[20], pancreatic ductal adenocarcinoma[10], and colorectal cancer[21]. We also showed that SERPINB5 was an upregulated gene in rectal cancer by mining public databases. Consistent with the result from public databases, our study confirmed that SERPINB5 was upregulated in rectal cancer tissues and cell lines. SERPINB5 has been recently identified as the most significantly upregulated gene among those related to cell motility in rectal cancer[11]. The upregulation of nucleocytoplasmic SERPINB5 is also significantly related to aggressive tumor features including tumor budding and tumor invasion and metastasis in colorectal cancer[22]. Recently, an in-depth study demonstrated that SERPINB5 promotes colorectal cancer invasion and migration by increasing epithelial-mesenchymal transition and angiogenesis[23]. These previous studies suggest that SERPINB5 is involved in tumor metastasis in colorectal cancer and rectal cancer. To date, the exact regulatory role of SERPINB5 in rectal cancer has rarely been discussed. Our in-depth studies illustrated that SERPINB5 overexpression substantially facilitated rectal cell proliferation and invasion and inhibited apoptosis. By contrast, SERPINB5 knockdown showed the opposite results. Notably, current evidence demonstrates that the strong expression of SERPINB5 in tumors is remarkably associated with a poor prognosis in patients with colorectal cancer[24]. High SERPINB5 expression is related to poor outcome after metastasis to local lymph nodes, and SERPINB5 serve as a marker for early recurrence in primary stage III and IV colorectal cancer[25]. Moreover, upregulated SERPINB5 expression is related to adverse clinicopathological features, including concurrent chemoradiotherapy resistance in patients with rectal cancer[11]. Based on these findings, SERPINB5 can serve as a promising prognostic indicator for patients with rectal cancer in the clinic. Additionally, our results showed that SERPINB5 knockdown retarded the tumor growth of rectal cancer in xenograft mice. This provided the evidence for the application of therapeutic methods targeting SERPINB5 in the clinic.
HSP90AA1 is involved in maintaining the stability and homeostasis of oncoproteins, which are essential for tumorigenesis. Emerging evidence has revealed that HSP90AA1 is overexpressed in multiple cancer types and predicts poor prognosis. For instance, plasma HSP90AA1 could conveniently predict the risk of distant metastasis for breast cancer[26]. HSP90AA1 level is upregulated in tumor tissues from patients with HNSC and is related to disease progression and poor overall survival[27]. Furthermore, high HSP90AA1 expression in tissues from patients with lung cancer indicate poor overall survival. HSP90AA1 inhibition promotes lung cancer cell apoptosis and suppresses proliferation and tumor growth in xenograft mouse models[28]. Importantly, HSP90AA1 expression is substantially overexpressed in colorectal cancer tissues. this expression pattern is related to poor prognosis and might act as an prognostic indicator[29]. Zhang et al[30] suggested that downregulation of HSP90AA1 inhibited the malignant proliferation and migration of colorectal cancer. Moreover, HSP90AA1 could serve as an effective biomarker to predict prognose and the sensitivity of neo
Based on the previous findings, SERPINB5 can be used as a promising diagnostic and prognostic indicator for rectal cancer in the clinic. Moreover, our results suggested that SERPINB5 knockdown inhibited cancer cell proliferation and metastasis and suppressed tumor growth in xenograft mice. These findings provide a promising therapeutic target for rectal cancer and an experimental basis for the application of therapeutic methods targeting SERPINB5 in the clinic. Therefore, the anticancer effects of SERPINB5 inhibition requires urgent exploration in clinical trials, considerably contributing to the development of rectal cancer treatment.
The present study still has some limitations. First, the limited sample size of patients with rectal cancer and insufficient sample sizes for in vitro and in vivo experiments may affect the generalizability and accuracy of our results. Second, the roles and mechanisms of SERPINB5 in rectal cancer were not fully investigated in this study. Third, the use of SERPINB5 as a prognostic biomarker for rectal cancer has not been proved in our study. In future studies, we intend to increase the sample size of patients and analyze the expression and prognostic significance of SERPINB5 for rectal cancer in the clinic. Moreover, other possible SERPINB5-mediated molecular mechanisms in rectal cancer will be investigated to provide more theoretical basis for the application of therapeutic methods targeting SERPINB5 in the clinic.
Our study revealed that SERPINB5 and HSP90AA1 were upregulated in rectal cancer tissues and cell lines. SERPINB5 knockdown inhibited proliferation and invasion and enhanced apoptosis in rectal cancer cells. SERPINB5 knockdown retarded rectal cancer tumor growth in xenograft mice. Mechanistically, SERPINB5 augmented rectal cancer progression through interacting with HSP90AA1. Our study may provide a promising target for rectal cancer treatment.
| 1. | Kang L, Chen YG, Zhang H, Zhang HY, Lin GL, Yang YC, Chen WH, Luo SL, Chen N, Tong WD, Shen ZL, Xiong DH, Xiao Y, Zhang ZT, Wang JP. Transanal total mesorectal excision for rectal cancer: a multicentric cohort study. Gastroenterol Rep (Oxf). 2020;8:36-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Suresh RS, Garcia LE, Gearhart SL. Young-Onset Rectal Cancer: Is It for Real? Adv Surg. 2024;58:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Keller DS, Reif de Paula T, Kiran RP. Ready for the National Accreditation Programs for Rectal Cancer? Auditing rectal cancer outcomes in the United States. Colorectal Dis. 2019;21:1213-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lee GC, Bordeianou LG, Francone TD, Blaszkowsky LS, Goldstone RN, Ricciardi R, Kunitake H, Qadan M. Superior pathologic and clinical outcomes after minimally invasive rectal cancer resection, compared to open resection. Surg Endosc. 2020;34:3435-3448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Conde-Muíño R, Cuadros M, Zambudio N, Segura-Jiménez I, Cano C, Palma P. Predictive Biomarkers to Chemoradiation in Locally Advanced Rectal Cancer. Biomed Res Int. 2015;2015:921435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Teoh SS, Whisstock JC, Bird PI. Maspin (SERPINB5) is an obligate intracellular serpin. J Biol Chem. 2010;285:10862-10869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 689] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 8. | Endsley MP, Zhang M. Investigating maspin in breast cancer progression using mouse models. Methods Enzymol. 2011;499:149-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Wang XF, Liang B, Zeng DX, Lei W, Chen C, Chen YB, Huang JA, Gu N, Zhu YH. The roles of MASPIN expression and subcellular localization in non-small cell lung cancer. Biosci Rep. 2020;40:BSR20200743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Tian C, Öhlund D, Rickelt S, Lidström T, Huang Y, Hao L, Zhao RT, Franklin O, Bhatia SN, Tuveson DA, Hynes RO. Cancer Cell-Derived Matrisome Proteins Promote Metastasis in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020;80:1461-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Chang IW, Liu KW, Ragunanan M, He HL, Shiue YL, Yu SC. SERPINB5 Expression: Association with CCRT Response and Prognostic Value in Rectal Cancer. Int J Med Sci. 2018;15:376-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Szczuka I, Wierzbicki J, Serek P, Szczęśniak-Sięga BM, Krzystek-Korpacka M. Heat Shock Proteins HSPA1 and HSP90AA1 Are Upregulated in Colorectal Polyps and Can Be Targeted in Cancer Cells by Anti-Inflammatory Oxicams with Arylpiperazine Pharmacophore and Benzoyl Moiety Substitutions at Thiazine Ring. Biomolecules. 2021;11:1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Condelli V, Crispo F, Pietrafesa M, Lettini G, Matassa DS, Esposito F, Landriscina M, Maddalena F. HSP90 Molecular Chaperones, Metabolic Rewiring, and Epigenetics: Impact on Tumor Progression and Perspective for Anticancer Therapy. Cells. 2019;8:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Ryu SW, Stewart R, Pectol DC, Ender NA, Wimalarathne O, Lee JH, Zanini CP, Harvey A, Huibregtse JM, Mueller P, Paull TT. Proteome-wide identification of HSP70/HSC70 chaperone clients in human cells. PLoS Biol. 2020;18:e3000606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S. Heat Shock Proteins and Cancer. Trends Pharmacol Sci. 2017;38:226-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 16. | Tian Y, Wang C, Chen S, Liu J, Fu Y, Luo Y. Extracellular Hsp90α and clusterin synergistically promote breast cancer epithelial-to-mesenchymal transition and metastasis via LRP1. J Cell Sci. 2019;132:jcs228213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 17. | Kim D, Moon JW, Min DH, Ko ES, Ahn B, Kim ES, Lee JY. AHA1 regulates cell migration and invasion via the EMT pathway in colorectal adenocarcinomas. Sci Rep. 2021;11:19946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64587] [Article Influence: 16146.8] [Reference Citation Analysis (176)] |
| 19. | Kim J, Jang KT, Kim KH, Park JW, Chang BJ, Lee KH, Lee JK, Heo JS, Choi SH, Choi DW, Rhee JC, Lee KT. Aberrant maspin expression is involved in early carcinogenesis of gallbladder cancer. Tumour Biol. 2010;31:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Ito Y, Yoshida H, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Matsuura N, Kuma K, Miyauchi A. Maspin expression is directly associated with biological aggressiveness of thyroid carcinoma. Thyroid. 2004;14:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Gurzu S, Jung I. Subcellular Expression of Maspin in Colorectal Cancer: Friend or Foe. Cancers (Basel). 2021;13:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Elkady N, Allam DM. The Role of Galectin3, Tubulinβ, and Maspin in Promoting Tumor Budding in Colorectal Carcinoma and Their Clinical Implications. Appl Immunohistochem Mol Morphol. 2024;32:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Liu BX, Xie Y, Zhang J, Zeng S, Li J, Tao Q, Yang J, Chen Y, Zeng C. SERPINB5 promotes colorectal cancer invasion and migration by promoting EMT and angiogenesis via the TNF-α/NF-κB pathway. Int Immunopharmacol. 2024;131:111759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 24. | Baek JY, Yeo HY, Chang HJ, Kim KH, Kim SY, Park JW, Park SC, Choi HS, Kim DY, Oh JH. Serpin B5 is a CEA-interacting biomarker for colorectal cancer. Int J Cancer. 2014;134:1595-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Snoeren N, Emmink BL, Koerkamp MJ, van Hooff SR, Goos JA, van Houdt WJ, de Wit M, Prins AM, Piersma SR, Pham TV, Belt EJ, Bril H, Stockmann HB, Meijer GA, van Hillegersberg R, Holstege FC, Jimenez CR, Fijneman RJ, Kranenburg OW, Rinkes IH. Maspin is a marker for early recurrence in primary stage III and IV colorectal cancer. Br J Cancer. 2013;109:1636-1647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Liu H, Zhang Z, Huang Y, Wei W, Ning S, Li J, Liang X, Liu K, Zhang L. Plasma HSP90AA1 Predicts the Risk of Breast Cancer Onset and Distant Metastasis. Front Cell Dev Biol. 2021;9:639596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Fan G, Tu Y, Wu N, Xiao H. The expression profiles and prognostic values of HSPs family members in Head and neck cancer. Cancer Cell Int. 2020;20:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Niu M, Zhang B, Li L, Su Z, Pu W, Zhao C, Wei L, Lian P, Lu R, Wang R, Wazir J, Gao Q, Song S, Wang H. Targeting HSP90 Inhibits Proliferation and Induces Apoptosis Through AKT1/ERK Pathway in Lung Cancer. Front Pharmacol. 2021;12:724192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Zhang S, Guo S, Li Z, Li D, Zhan Q. High expression of HSP90 is associated with poor prognosis in patients with colorectal cancer. PeerJ. 2019;7:e7946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Zhang M, Peng Y, Yang Z, Zhang H, Xu C, Liu L, Zhao Q, Wu J, Wang H, Liu J. DAB2IP down-regulates HSP90AA1 to inhibit the malignant biological behaviors of colorectal cancer. BMC Cancer. 2022;22:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | Jiang XF, Zhang BM, Du FQ, Guo JN, Wang D, Li YE, Deng SH, Cui BB, Liu YL. Exploring biomarkers for prognosis and neoadjuvant chemosensitivity in rectal cancer: Multi-omics and ctDNA sequencing collaboration. Front Immunol. 2022;13:1013828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |