Published online Mar 21, 2025. doi: 10.3748/wjg.v31.i11.103178
Revised: January 12, 2025
Accepted: February 25, 2025
Published online: March 21, 2025
Processing time: 122 Days and 15.6 Hours
Fontan-associated liver disease (FALD) often occurs in patients with single-ventricle physiology following Fontan surgery, and ranges from liver congestion to cirrhosis. The assessment of the severity of FALD using noninvasive methods is challenging. However, transient elastography (TE) may be useful for the non
To evaluate the role of TE in the diagnosis of FALD and its association with clinically relevant events.
This retrospective single-center study (Hospital Universitario La Paz, Madrid), including 91 post-Fontan patients aged > 18 years old. Laboratory and ultrasound findings, and liver stiffness measurements (LSM) by TE (FibroScan®) were assessed. FALD was defined using ultrasound criteria (hepatomegaly, liver surface nodularity, parenchymal heterogeneity, hyperechoic lesions, splenomegaly, collaterals) and advanced FALD was defined according to the European Association for the Study of the Liver-European Reference Network statement (esophageal varices, portosystemic shunts, ascites, splenomegaly). Clinically relevant events included heart or heart-liver transplantation indication, hepatocellular carcinoma, and all-cause mortality.
Patient characteristics were: 60.4% male; Mean age, 33.3 ± 8.2 years; Mean elapsed time since surgery, 24.3 ± 7.7 years; 89% with FALD; 73% with advanced FALD. LSM by TE was associated with FALD [odds ratio (OR) = 1.34; 95% confidence interval (95%CI): 1.10-1.64; P = 0.003] and advanced FALD (OR = 1.10; 95%CI: 1.01-1.19; P = 0.023). Areas under the curve (AUC) were 0.905 and 0.764 for FALD and advanced FALD, respectively. FALD cut-off values comprised: Optimal, 20 kPa (sensitivity: 92.3%; specificity: 80.0%); Rule-out, 15 kPa (sensitivity: 96.9%); Rule-in, 25 kPa (specificity: 100%). A FALD algorithm was proposed based on LSM by TE and elapsed time since surgery (AUC: 0.877; sensitivity, 95.4%; specificity, 80.0%; positive predictive value, 96.9%; negative predictive value, 72.7%). LSM by TE was associated with clinically relevant events (OR = 1.07; 95%CI: 1.01-1.13; P = 0.021) and all-cause mortality (OR = 1.23; 95%CI: 1.02-1.47; P = 0.026).
In adult patients post-Fontan surgery, TE is a useful noninvasive method for FALD diagnosis. The association between LSM by TE and clinically relevant events suggests a role in prognosis.
Core Tip: There was a significant association between liver stiffness measurement (LSM) by transient elastography (TE) and Fontan-associated liver disease (FALD). We obtained areas under the curve (AUC) of 0.905 for FALD and 0.764 for advanced FALD. The optimal cut-off values were 20 kPa for FALD and 25 kPa for advanced FALD. An algorithm for FALD was proposed based on LSM by TE and elapsed time since Fontan surgery with an AUC of 0.877. We also demonstrated an association between LSM by TE and clinically relevant events (heart or heart-liver transplantation indication, hepatocellular carcinoma, and all-cause mortality).
- Citation: Cuadros M, Abadía M, Castillo P, Martín-Arranz MD, Gonzalo N, Romero M, García-Sánchez A, García-Samaniego J, Olveira A, Ruiz-Cantador J, González-Fernández Ó, Ponz I, Merás P, Merino C, Rodríguez-Chaverri A, Balbacid E, Froilán C. Role of transient elastography in the diagnosis and prognosis of Fontan-associated liver disease. World J Gastroenterol 2025; 31(11): 103178
- URL: https://www.wjgnet.com/1007-9327/full/v31/i11/103178.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i11.103178
The Fontan procedure is the main palliative surgical approach for improving survival in patients with congenital heart disease (CHD) and single-ventricle physiology[1]. Hemodynamic modifications after this surgery lead to splanchnic congestion and a lack of pulsatility, resulting in liver congestion and the development of chronic liver disease, portal hypertension, and its complications[2,3].
Fontan-associated liver disease (FALD) refers to the structural and dynamic changes that affect the liver following the Fontan surgery[4]. Observational studies have demonstrated that liver fibrosis can occur up to 10 years post-Fontan surgery, and many adult patients develop end-stage cirrhosis[5]. Recently, the European Association for the Study of the Liver (EASL) and the European Reference Network (ERN) on rare liver diseases proposed a definition for “advanced FALD”, comprising patients with the following portal hypertension signs: Esophageal varices, portosystemic shunts, ascites, or splenomegaly[6]. The noninvasive assessment of liver fibrosis in patients post-Fontan surgery is challenging. Serological methods have not been validated in this setting, and imaging features of cirrhosis, such as a nodular liver surface or heterogeneous parenchyma, may not predict liver fibrosis in such patients[7].
Transient elastography (TE) is the most widely used noninvasive technique for staging liver fibrosis and has been validated for many etiologies such as viral hepatitis, alcohol-related liver disease, and non-alcoholic fatty liver disease[8]. TE could be a particularly relevant method for patients that have undergone Fontan surgery in whom invasive procedures may have a higher risk of complications. However, in these patients, liver stiffness may be increased due to liver congestion, and determining the components of fibrosis is complicated. Few studies have addressed the role of TE in FALD, and the cut-off values have not been validated[9,10]. In 2024, Téllez et al[11] developed the FonLiver risk score to predict severe liver fibrosis using TE and platelet count, with an area under the receiver operating characteristic curve (AUROC) of 0.810. TE also plays a relevant prognostic role in the prediction of decompensation and liver-related death in many patients with liver diseases[12]. However, this has not been demonstrated in Fontan patients. Further research is required to determine the usefulness of TE in predicting clinical outcomes and monitoring the course of heart disease in such patients.
The aim of our study was to assess the association between liver stiffness measurement (LSM) by TE and FALD, and determine the cut-off values for FALD and advanced FALD. In addition, we evaluated the association between LSM by TE and clinically relevant events (heart or heart-liver transplantation indication, hepatocellular carcinoma, and all-cause mortality) in patients post-Fontan surgery.
We included 91 adult patients with single-ventricle physiology repaired by Fontan surgery in an observational single-center study (Hospital Universitario La Paz, Madrid, Spain) from January 2017 to November 2023. This study was approved by the Research Ethics Board of the Hospital Universitario La Paz (PI-5510).
The inclusion criteria were as follows: Patients older than 18 under follow-up in the adult CHD unit of our center whose medical reports were available. Patients with liver disease other than FALD were excluded.
We collected data on the clinical history of each patient, including anthropometric parameters, type of CHD, type of Fontan surgery, elapsed time since Fontan surgery, cardiological characteristics, laboratory data, serological and clinical scores, liver features on imaging tests, liver TE, and clinical outcomes such as heart and combined heart-liver transplantation indication, hepatocellular carcinoma, and all-cause mortality.
The measured laboratory parameters included hemoglobin, platelet count, prothrombin time, international normalized ratio (INR), total/direct bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, gamma-glutamyl transpeptidase (GGT), albumin, creatinine, sodium, total cholesterol, N-terminal fragment brain natriuretic peptides, and alpha-fetoprotein levels.
We calculated the following parameters. Liver fibrosis scores: AST to platelet ratio index (APRI), fibrosis-4 index (FIB-4), and Forns index; Prognostic scores: Model for end-stage liver disease (MELD), MELD-sodium (MELD-Na), MELD excluding INR (MELD-XI), and Child-Pugh; and Portal hypertension score: Varices, ascites, splenomegaly, or thrombocytopenia (VAST). These parameters were calculated as follows:
APRI: AST (/upper limit normal)/platelet count (109 /L) × 100.
FIB-4: Age (years) × AST (U/L)/platelet count (109 /L) × [ALT (U/L)1/2].
Forns index: 7.811 - 3.131 × Ln [platelet count (109/L)] + 0.781 × Ln [GGT (IU/L) + 3.467 × Ln [age (years)] - 0.014 [cholesterol (mg = dL)].
MELD: 9.6 Ln [serum creatinine (mg/dL)] + 3.8 Ln [(total serum bilirubin (mg/dL)) + 11.2 Ln (INR) + 6.4.
MELD-XI: 11.76 × Ln [serum creatinine (mg/dL)] + 5.11 × Ln [total serum bilirubin (mg/dL)] + 9.44.
VAST: One point each for varices, ascites, splenomegaly, and thrombocytopenia[13].
Abdominal ultrasounds (US) were performed by expert staff using a Canon Aplio i800 US system (Canon Medical Systems). The liver findings registered comprised heterogeneous echogenicity, nodular surface, hepatomegaly (diameter > 160 mm), abnormal caudate lobe (> 35 mm), and presence of liver nodules (including number and size). Signs of portal hypertension signs included portal vein dilation (> 13 mm), splenomegaly (diameter > 125 mm, or area > 55 cm2), collateral circulation, portosystemic shunts, and ascites.
LSM were performed by TE (FibroScan®, Echosens, Paris, France), applying a standardized procedure, and using medium or extra-large probes. The median value among 10 valid measurements was calculated, obtaining an inter
FALD was defined using US criteria when one of the following characteristics was observed: Hepatomegaly, liver surface nodularity, parenchymal heterogeneity, hyperechoic lesions, splenomegaly, or collaterals. Advanced FALD was defined based on the EASL-ERN criteria when one of the following conditions presented: Esophageal varices, portosystemic shunts, ascites, or splenomegaly.
Qualitative variables were summarized as frequencies and percentages, and quantitative variables were presented as measures of central tendency (mean and median) and dispersion (standard deviation and 25th and 75th percentiles). Differences in the percentage distribution of qualitative variables were analyzed using the χ2 test or Fisher’s exact test, as appropriate. The normality of continuous variables was tested using the Kolmogorov-Smirnov test, and comparisons between groups were performed using Student’s t test, Mann-Whitney U test, or one-way analysis of variance or Kruskal-Wallis test, depending on the normality of the variable and the number of groups compared. Multiple comparisons were performed using the Bonferroni method.
Receiver operating characteristic (ROC) analyses were conducted to evaluate the diagnostic capacity of the imaging tests and other indicators (analytical and non-analytical) for FALD, advanced FALD, and prognostic factors (mortality, transplantation indication, and/or hepatocarcinoma). An AUROC of 80%-90% was considered acceptable, and > 90% was considered excellent. Optimal cut-off values were determined according to the Youden index and their applicability, as well as “rule-out” and “rule-in” points based on sensitivity and specificity values equal to or greater than 90%. In the indeterminate areas defined by the “rule-in” and “rule-out” values, new ROC analyses were performed to maximize the diagnostic capacity of the tests. The final diagnostic algorithms were constructed by considering the best values of sensitivity, specificity, positive/negative predictive values (PPV/NPV), positive/negative likelihood ratios, and the percentage of patients correctly classified by the model (power or accuracy of the test).
The identification of predictive factors for FALD, advanced FALD, and other prognostic events (mortality, trans
The IBM Statistical Product and Service Solutions statistics 27.0 (IBM Corp., Armonk, NY, United States) package was used for the statistical analysis, and a statistical significance level of P < 0.05 (two-tailed) was considered for all tests.
A total of 91 adult patients who had undergone Fontan surgery were analyzed. Fifty-five (60.4%) were male, mean patient age was 33.3 ± 8.2 years, and the mean elapsed time since Fontan surgery was 24.3 ± 7.7 years. FALD was observed in 81 out of 91 patients (89%). Data on advanced FALD criteria were available for 74 patients. Fifty-four out of 74 patients presented advanced FALD (73%). Table 1 and Table 2 show the characteristics of the study population comparing non-FALD with FALD and non-advanced FALD with advanced FALD.
| General and cardiologic characteristics | Total (n = 91) | Non-FALD (n = 10) | FALD (n = 81) | P value |
| Male | 55 (60.4) | 6 (60.0) | 49 (60.5) | NS |
| Age, years | 33.3 ± 8.2 | 31.8 ± 9.7 | 33.4 ± 8.0 | NS |
| Age, years, median (IQR) | 32.0 (27.0-38.0) | 30.0 (22.0-37.0) | 32.0 (28.0-38.0) | |
| BMI, kg/m2, median (IQR) | 23.1 (20.4-25.8) | 25.7 (24.3-26.3) | 22.7 (20.3-25.0) | 0.048 |
| Time since Fontan, years | 24.3 ± 7.7 | 19.3 ± 11.1 | 24.8 ± 7.1 | 0.074 |
| Time since Fontan, years, median (IQR) | 23.0 (19.0-28.0) | 19.0 (9.0-23.0) | 23.0 (19.5-28.0) | |
| Type of congenital heart disease | NS | |||
| Pulmonary atresia or stenosis | 50 (54.9) | 7 (70.0) | 43 (53.1) | |
| Tricuspid atresia | 39 (42.9) | 6 (60.0) | 33 (40.7) | |
| Transposition of great arteries | 34 (37.4) | 5 (50.0) | 29 (35.0) | |
| Doble outlet right ventricle | 15 (16.5) | 0 (0.0) | 15 (18.5) | |
| Doble inlet left ventricle | 13 (14.3) | 0 (0.0) | 13 (16.0) | |
| Left heart/ventricle hypoplasia | 10 (11.0) | 0 (0.0) | 10 (12.4) | |
| Right heart/ventricle hypoplasia | 12 (13.2) | 1 (10.0) | 11 (13.6) | |
| Mitral atresia | 8 (8.8) | 0 (0.0) | 8 (9.9) | |
| Type of surgery | 0.077 | |||
| Atriopulmonary | 19 (21.1) | 0 (0.0) | 19 (23.5) | |
| Bicavopulmonary | ||||
| Intra-atrial lateral conduit | 8 (8.9) | 2 (22.2) | 6 (7.4) | |
| Extracardiac conduit | 63 (70.0) | 7 (77.8) | 56 (69.1) | |
| Fontan conduit stenosis | 35 (38.9) | 0 (0.0) | 35 (43.8) | 0.006 |
| Oxygen saturation, %, median (IQR) | 93.0 (91.0-95.0) | 94.5 (93.0-96.0) | 93.0 (91.0-95.0) | NS |
| Laboratory investigations and serological/clinical scores | ||||
| Hemoglobin, g/dL, median (IQR) | 16.5 (14.8-17.6) | 16.6 (15.4-17.1) | 16.5 (14.5-17.6) | NS |
| Platelet count, × 103/L, median (IQR) | 135.0 (115.0-182.0) | 188.5 (162.0-209.0) | 131.0 (111.0-169.0) | 0.002 |
| Prothrombin, %, median (IQR) | 74.0 (50.0-83.0) | 83.0 (32.0-88.0) | 74.0 (55.0-82.0) | NS |
| INR, median (IQR) | 1.1 (1.1-1.4) | 1.1 (1.1-1.4) | 1.1 (1,1-1.4) | NS |
| AST, UI/L, median (IQR) | 28.0 (24.0-35.0) | 29.0 (22.0-31.0) | 28.0 (24.0-36.0) | NS |
| ALT, UI/L, median (IQR) | 33.0 (24.0-43.0) | 35.5 (24.0-42.0) | 32.0 (24.0-44.0) | NS |
| ALP, UI/L, median (IQR) | 82.0 (66.5-105.5) | 82.0 (70.0-89.0) | 81.5 (66.0-108.0) | NS |
| GGT, UI/L, median (IQR) | 78.0 (56.0-121.0) | 66.0 (44.0-110.0) | 79.0 (56.0-121.0) | NS |
| Total bilirubin, mg/dL, median (IQR) | 1.3 (0.9-2.1) | 1.2 (0.8-1.9) | 1.3 (0.9-2.1) | NS |
| Direct bilirubin, mg/dL, median (IQR) | 0.7 (0.6-1.0) | 0.5 (0.3-1.0) | 0.7 (0.6-1.0) | NS |
| Creatinine, mg/dL, median (IQR) | 0.8 (0.7-0.9) | 0.9 (0.7-1.0) | 0.8 (0.7-0.9) | NS |
| Sodium, mEq/L, median (IQR) | 140.0 (138.9-141.0) | 139.0 (139.0-140.0) | 140.0 (138.0-141.0) | NS |
| Albumin, g/dL, median (IQR) | 4.6 (4.4-4.9) | 4.7 (4.5-4.8) | 4.6 (4.3-4.9) | NS |
| Total cholesterol, mg/dL, median (IQR) | 140.0 (121.0-162.0) | 142.5 (138.0-163.0) | 139.0 (118.0-162.0) | NS |
| AFP, ng/mL, median (IQR) | 2.4 (1.5-4.2) | 2.5 (2.4-3.1) | 2.4 (1.5-4.2) | NS |
| NT-proBNP, pg/mL, median (IQR) | 217.0 (100.0-579.0) | 178.5 (60.5-455.5) | 234.0 (107.0-587.0) | NS |
| APRI, median (IQR) | 0.5 (0.4-0.7) | 0.4 (0.2-0.6) | 0.5 (0.4-0.7) | 0.030 |
| FIB-4, median (IQR) | 1.1 (0.9-1.6) | 0.8 (0.6-1.0) | 1.2 (0.9-1.8) | 0.017 |
| Forns index, median (IQR) | 5.7 (4.9-6.8) | 4.5 (3.5-5.2) | 5.8 (5.0-6.9) | 0.005 |
| MELD, median (IQR) | 9 (7-11) | 8 (7-10) | 9 (7-11) | NS |
| MELD-Na, median (IQR) | 10 (7-11) | 8 (7-10) | 10 (7-11) | NS |
| MELD-XI, median (IQR) | 11 (9-13) | 10 (9-13) | 11 (9-13) | NS |
| Child-Pugh | NS | |||
| A (5-6) | 84 (92.3) | 10 (100.0) | 74 (91.4) | |
| B (7-9) | 7 (7.7) | 0 (0.0) | 7 (8.6) | |
| VAST ≥ 2 | 42 (75.0) | 0 (0.0) | 42 (77.8) | < 0.001 |
| Ultrasonography and transient elastography | ||||
| Heterogenous liver echogenicity | 61 (67.8) | 0 (0.0) | 61 (76.3) | < 0.001 |
| Nodular liver surface | 55 (61.8) | 0 (0.0) | 55 (69.6) | < 0.001 |
| Hepatomegaly | 35 (39.3) | 0 (0.0) | 35 (44.3) | 0.005 |
| Abnormal caudate | 49 (55.7) | 0 (0.0) | 49 (62.8) | < 0.001 |
| Liver nodules | 38 (42.2) | 4 (40.0) | 34 (42.5) | NS |
| Portal vein dilation | 7 (8.9) | 0 (0.0) | 7 (9.0) | NS |
| Splenomegaly | 46 (50.5) | 0 (0.0) | 46 (58.8) | < 0.001 |
| Collateral circulation | 17 (18.9) | 0 (0.0) | 17 (21.3) | NS |
| Portosystemic shunts | 6 (6.7) | 0 (0.0) | 6 (7.6) | NS |
| Ascites, grade | NS | |||
| 0 | 75 (83.3) | 10 (100.0) | 65 (81.3) | |
| 1 | 10 (11.1) | 0 (0.0) | 10 (12.5) | |
| 2 | 5 (5.6) | 0 (0.0) | 5 (6.3) | |
| Liver stiffness, kPa, median (IQR) | 25.3 (20.3-33.0) | 14.6 (11.4-18.5) | 27.7 (21.9-34.3) | < 0.001 |
| General and cardiologic characteristics | Total (n = 74) | Non-advanced FALD (n = 20) | Advanced FALD (n = 54) | P value |
| Male | 45 (60.8) | 11 (55.0) | 34 (63.9) | NS |
| Age, years | 33.0 ± 7.5 | 32.2 ± 8.9 | 33.2 ± 7.0 | NS |
| Age, years, median (IQR) | 32.0 (28.0-38.0) | 32.5 (23.0-39.0) | 32.0 (28.0-37.0) | |
| BMI, kg/m2, median (IQR) | 22.4 (20.3-25.7) | 24.3 (20.3-26.0) | 22.2 (19.6-24.9) | NS |
| Time since Fontan, median (IQR) | 23.0 (19.0-27.5) | 21.0 (18.0-28.0) | 23.0 (20.0-27.0) | NS |
| Type of congenital heart disease | NS | |||
| Pulmonary atresia or stenosis | 41 (55.4) | 10 (50.0) | 31 (57.4) | |
| Tricuspid atresia | 31 (41.9) | 11 (55.0) | 20 (37.0) | |
| Transposition of great arteries | 26 (35.1) | 7 (35.0) | 19 (35.2) | |
| Doble outlet right ventricle | 12 (16.2) | 3 (15.0) | 9 (16.7) | |
| Doble inlet left ventricle | 8 (10.8) | 1 (5.0) | 7 (13.0) | |
| Left heart/ventricle hypoplasia | 10 (13.5) | 2 (10.0) | 8 (14.8) | |
| Right heart/ventricle hypoplasia | 12 (16.2) | 2 (10.0) | 10 (18.5) | |
| Mitral atresia | 7 (9.5) | 1 (5.0) | 6 (11.1) | |
| Type of surgery | NS | |||
| Atriopulmonary | 16 (21.9) | 3 (15.8) | 13 (24.1) | |
| Bicavopulmonary | ||||
| Intra-atrial lateral conduit | 6 (8.2) | 2 (10.5) | 4 (7.4) | |
| Extracardiac conduit | 51 (69.9) | 14 (73.7) | 37 (68.5) | |
| Fontan conduit stenosis | 30 (41.1) | 4 (20.0) | 26 (49.1) | 0.033 |
| Oxygen saturation, %, median (IQR) | 92.0 (91.0-95.0) | 94.0 (92.5-96.0) | 92.0 (90.0-94.0) | 0.007 |
| Laboratory investigations and serological/clinical scores | ||||
| Hemoglobin, g/dL, median (IQR) | 16.3 (14.5-17.6) | 16.2 (14.5-17.0) | 16.3 (14.5-17.6) | NS |
| Platelet count, × 103/L, median (IQR) | 130.0 (111.0-169.0) | 162.0 (142.0-198.0) | 120.0 (97.0-150.0) | < 0.001 |
| Prothrombin, %, median (IQR) | 73.0 (50.0-82.5) | 73.0 (34.5-87.9) | 72.5 (51.0-81.0) | NS |
| INR, median (IQR) | 1.1 (1.1-1.4) | 1.1 (1.1-1.4) | 1.2 (1.1-1.4) | NS |
| AST, UI/L, median (IQR) | 28.0 (23.5-33.0) | 29.0 (23.0-36.0) | 28.0 (23.5-32.0) | NS |
| ALT, UI/L, median (IQR) | 31.5 (23.5-43.0) | 34.0 (22.5-44.0) | 31.0 (24.0-42.0) | NS |
| ALP, UI/L, median (IQR) | 81.0 (66.0-108.0) | 73.5 (62.5-84.0) | 87.0 (66.0-114.0) | NS |
| GGT, UI/L, median (IQR) | 77.0 (54.5-124.0) | 62.5 (43.0-89.5) | 80.5 (60.0-140.0) | 0.025 |
| Total bilirubin, mg/dL, median (IQR) | 1.4 (0.9-2.1) | 0.9 (0.7-1.8) | 1.5 (1.0-2.2) | 0.030 |
| Direct bilirubin, mg/dL, median (IQR) | 0.7 (0.6-1.0) | 0.6 (0.4-1.0) | 0.7 (0.6-1.0) | NS |
| Creatinine, mg/dL, median (IQR) | 0.8 (0.7-0.9) | 0.9 (0.7-0.9) | 0.8 (0.7-0.9) | NS |
| Sodium, mEq/L, median (IQR) | 140.0 (138.9-141.0) | 140.0 (139.0-142.0) | 140.0 (138.0-141.0) | NS |
| Albumin, g/dL, median (IQR) | 4.6 (4.3-4.9) | 4.7 (4.5-4.8) | 4.6 (4.3-4.9) | NS |
| Total cholesterol, mg/dL, median (IQR) | 139.0 (122.0-161.5) | 142.5 (129.0-150.5) | 137.5 (118.5-163.5) | NS |
| AFP, ng/mL, median (IQR) | 2.4 (1.6-4.4) | 2.4 (2.0-3.1) | 2.3 (1.5-4.9) | NS |
| NT-proBNP, pg/mL, median (IQR) | 223.0 (100.0-579.0) | 181.0 (82.0-453.0) | 246.0 (107.0-737.0) | NS |
| APRI, median (IQR) | 0.51 (0.37-0.71) | 0.40 (0.33-0.63) | 0.53 (0.39-0.77) | 0.024 |
| FIB-4, median (IQR) | 1.1 (0.9-1.7) | 1.0 (0.7-1.3) | 1.2 (0.9-1.9) | 0.028 |
| Forns index, median (IQR) | 5.7 (5.0-6.8) | 5.0 (4.3-5.6) | 6.3 (5.4-7.2) | < 0.001 |
| MELD, median (IQR) | 9 (7-11) | 8 (7-9) | 10 (8-11) | 0.024 |
| MELD-Na, median (IQR) | 10 (8-12) | 7 (7-9) | 10 (8-12) | 0.007 |
| MELD-XI, median (IQR) | 12 (9-13) | 10 (9-13) | 12 (10-14) | 0.040 |
| Child-Pugh | 0.084 | |||
| A (5-6) | 67 (90.5) | 20 (100.0) | 47 (87.0) | |
| B (7-9) | 7 (9.5) | 0 (0.0) | 7 (13.0) | |
| VAST ≥ 2 | 42 (75.0) | 0 (0.0) | 42 (93.4) | < 0.001 |
| Ultrasonography and transient elastography | ||||
| Heterogenous liver echogenicity | 46 (63.0) | 7 (35.0) | 39 (73.6) | 0.006 |
| Nodular liver surface | 44 (60.3) | 6 (30.0) | 38 (71.7) | 0.003 |
| Hepatomegaly | 30 (40.5) | 3 (15.0) | 27 (50.0) | 0.008 |
| Abnormal caudate | 42 (57.5) | 5 (25.0) | 37 (69.8) | 0.001 |
| Portal vein dilation | 7 (9.6) | 1 (5.0) | 6 (11.3) | NS |
| Splenomegaly | 46 (62.2) | 0 (0.0) | 46 (85.2) | < 0.001 |
| Collateral circulation | 17 (23.0) | 0 (0.0) | 17 (31.5) | 0.004 |
| Portosystemic shunts | 6 (8.1) | 0 (0.0) | 6 (11.1) | NS |
| Ascites, grade | NS | |||
| 0 | 59 (79.7) | 19 (95.9) | 40 (74.1) | |
| 1 | 10 (13.5) | 1 (5.0) | 9 (16.7) | |
| 2 | 5 (6.8) | 0 (0.0) | 5 (9.3) | |
| Liver stiffness, kPa, median (IQR) | 26.8 (20.3-34.3) | 19.0 (13.0-30.0) | 29.5 (22.6-36.4) | 0.001 |
The most common CHD was pulmonary atresia or stenosis (54.9%), and the most prevalent technique was the extracardiac conduit variant (70%). There were no significant differences in the occurrence of FALD with respect to CHD or type of surgery. Stenosis of the Fontan conduit was observed in 35 patients (38.9%), and it was significantly associated with FALD and advanced FALD (P < 0.05).
Platelet counts were significantly lower in patients with FALD and advanced FALD (P < 0.05). Liver fibrosis scores of the APRI, FIB-4, and Forns index were significantly higher in patients with FALD and advanced FALD, than in those without. The prognostic scores of MELD, MELD-Na, and MELD-XI were also significantly higher in patients with advanced FALD than in those without (P < 0.05).
Heterogeneous liver echogenicity was the most prevalent imaging finding (67.8%). Median LSM by TE in the total population was 25.3 kPa (IQR: 20.3-33.0 kPa). Median LSM was significantly higher in patients with FALD than in those without FALD (27.7 vs 14.6 kPa, P < 0.05), and in patients with advanced FALD compared to those with non-advanced FALD (29.5 vs 19.0, P < 0.05).
Factors associated with FALD: The univariate analysis demonstrated significant associations between FALD and the following factors: Elapsed time since Fontan surgery, conduit stenosis, platelet count, FIB-4, Forns index, and LSM. Multivariate analysis included the variables with statistical significance in the univariate analysis and of clinical relevance. FALD was significantly associated with LSM and Forns index in the multivariate analysis (Table 3).
| Variable | Multivariate analysis | |
| OR (95%CI) | P value | |
| Age | NS | |
| Time since Fontan surgery | NS | |
| Forns index | 2.59 (1.04-6.46) | 0.042 |
| Liver stiffness measurement | 1.34 (1.10-1.64) | 0.003 |
Factors associated with advanced FALD: The univariate analysis revealed significant associations between FALD and the following factors: Conduit stenosis, platelet count, GGT levels, APRI, FIB-4, Forns index, and LSM. In the multivariate analysis, advanced FALD was significantly associated with LSM and oxygen saturation (Table 4).
| Variable | Multivariate analysis | |
| OR (95%CI) | P value | |
| Age | NS | |
| Time since Fontan surgery | NS | |
| Conduit stenosis | NS | |
| Oxygen saturation | 0.68 (0.51-0.92) | 0.013 |
| Liver stiffness measurement | 1.10 (1.01-1.19) | 0.023 |
LSM by TE was significantly higher in patients with FALD than those without FALD, with a median value of 27.7 kPa (IQR: 21.9-34.3 kPa) vs 14.6 kPa (IQR: 11.4-18.5 kPa) (P < 0.001). LSM was significantly associated with FALD in the univariate and multivariate analysis [odds ratio (OR) = 1.34; 95% confidence interval (95%CI): 1.10-1.64; P = 0.003].
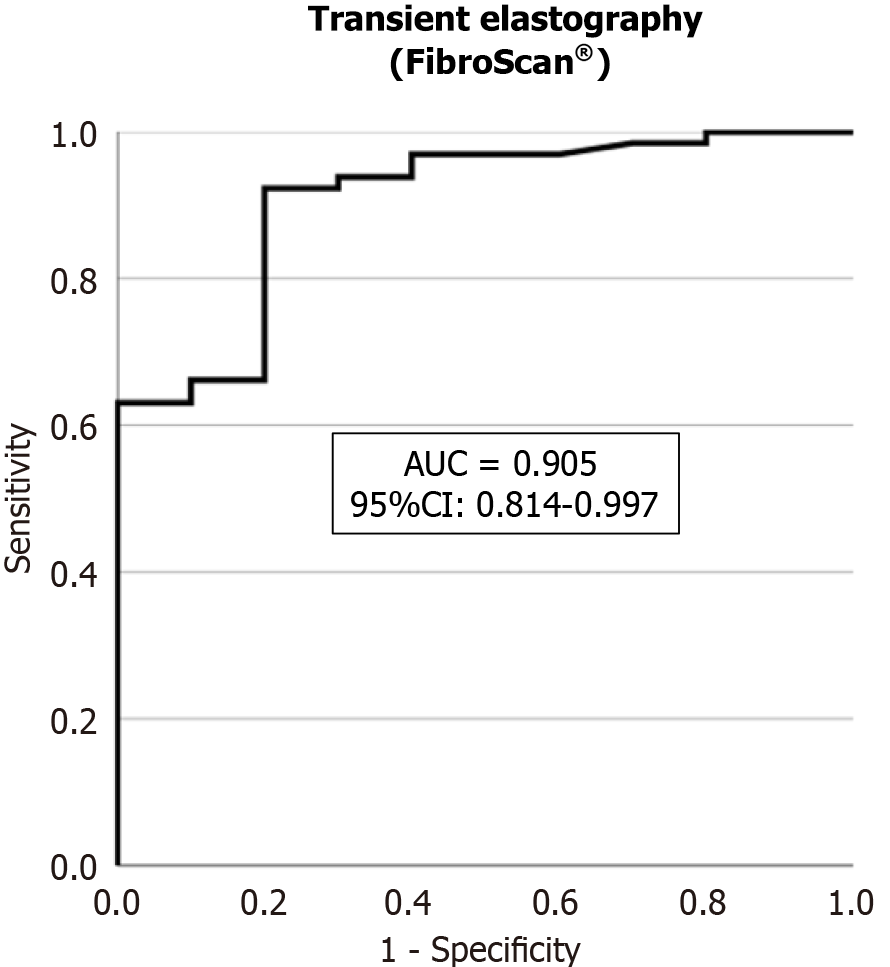
The AUROC of LSM by TE for FALD was 0.905 (95%CI: 0.814-0.997, P < 0.001) (Figure 1). We defined different LSM cut-off values for FALD (Table 5). The optimal cut-off value was 20 kPa (sensitivity, 92.3%; specificity, 80.0%; PPV, 96.8%; NPV, 61.5%), the cut-off value to rule-out FALD was 15 kPa (sensitivity, 96.9%; specificity, 50.0%), and the cut-off value to rule-in FALD was 25 kPa (specificity, 100%; sensitivity, 61.5%).
| LSM cut-off value | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Rule-out cut-off | < 15 | 0.969 | 0.500 | 0.926 | 0.714 | 0.907 |
| Optimal cut-off | 20 | 0.923 | 0.800 | 0.968 | 0.615 | 0.907 |
| Rule-in cut-off | ≥ 25 | 0.615 | 1.000 | 1.000 | 0.286 | 0.667 |
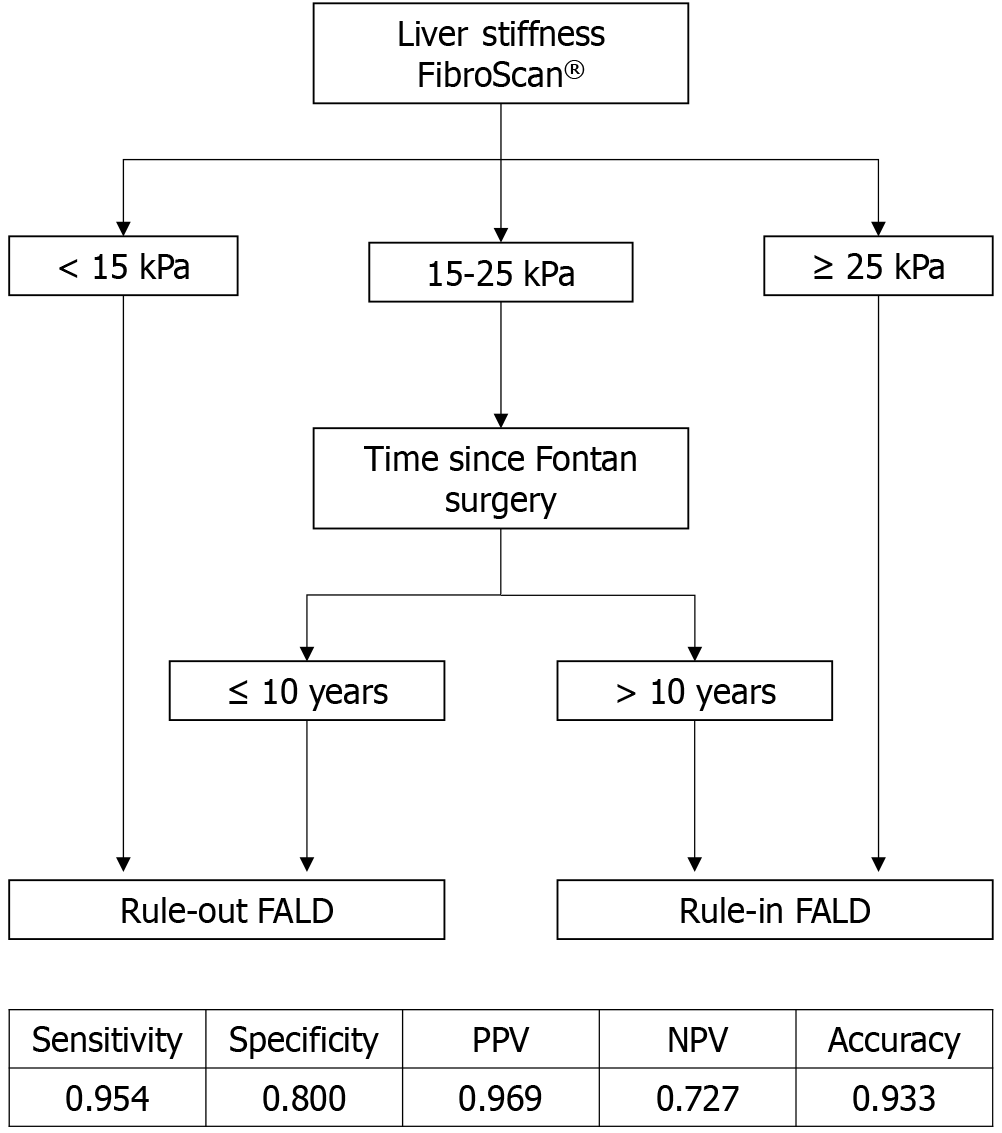
We also obtained an algorithm based on the combination of LSM by TE and elapsed time since Fontan surgery, which allowed us to rule-in and rule-out FALD with an AUROC of 0.877 (95%CI: 0.727-1.000; P < 0.001), with 95.4% sensitivity, 80.0% specificity, 96.9% PPV, 72.7% NPV, and 93.3% accuracy (Figure 2). According to this algorithm, we could rule-out FALD when LSM was < 15 kPa, and between 15-25 kPa, when the elapsed time since Fontan surgery was ≤ 10 years. We could rule-in FALD when LSM was ≥ 25 kPa, and between 15-25 kPa, when the elapsed time since Fontan surgery was > 10 years.
Patients with advanced FALD had a higher LSM by TE than those without FALD, with a median value of 29.5 kPa (22.4-36.6 kPa) vs 19.0 kPa (13.0-30.0 kPa) (P = 0.001). LSM by TE was significantly associated with advanced FALD in univariate and multivariate analysis (OR = 1.10; 95%CI: 1.01-1.19; P= 0.023).
The AUROC for advanced FALD was 0.764 (95%CI: 0.624-0.905; P = 0.001). The optimal LSM cut-off value for advanced FALD was 25 kPa (sensitivity, 69.6%; specificity, 68.4%; PPV, 84.2%; NPV, 48.1%). The cut-off value to rule-out advanced FALD was 20 kPa (sensitivity, 95.7%; specificity, 47.4%), and the cut-off value to rule-in advanced FALD was 40 kPa (specificity, 94.8%; sensitivity, 19.6%) (Table 6).
| LSM cut-off value | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Rule-out cut-off | < 20 | 0.957 | 0.474 | 0.815 | 0.818 | 0.815 |
| Optimal cut-off | 25 | 0.696 | 0.684 | 0.842 | 0.481 | 0.691 |
| Rule-in cut-off | ≥ 40 | 0.196 | 0.947 | 0.900 | 0.327 | 0.415 |
We assessed the following clinically relevant events: Heart or combined heart-liver transplantation indication, hepatocellular carcinoma, and all-cause mortality. There were a total of 26 events: 15 patients in the cohort were evaluated for transplantation (11 for heart transplantation and 4 for heart-liver transplantation), 3 patients were diagnosed with hepatocellular carcinoma, and 8 patients died during the study period. All patients who presented with clinically relevant events had been diagnosed with FALD; However, none of the deaths were liver-related. The causes of death were massive intraoperative bleeding during heart transplantation (3 patients), infection (1 patient), ischemic heart disease (1 patient), stroke (1 patient), and unknown (2 patients).
TE and mortality: LSM by TE was significantly higher in patients who died, with a median value of 44.6 kPa (34.6-62.9 kPa) vs 25 kPa (20.2-32.2 kPa) (P < 0.05). Multivariate analysis showed a significant association between LSM by TE and mortality (OR = 1.23; 95%CI: 1.02-1.47; P = 0.026) (Table 7). Additionally, elapsed time since Fontan surgery was associated with mortality events in the multivariate analysis (OR = 1.36; 95%CI: 1.04-1.79; P = 0.026).
| Variable | Multivariate analysis | |
| OR (95%CI) | P value | |
| Time since Fontan surgery | 1.36 (1.04-1.79) | 0.026 |
| Liver stiffness measurement | 1.23 (1.02-1.47) | 0.026 |
TE and heart/heart-liver transplantation indication, hepatocellular carcinoma, and mortality: LSM by TE was sig
| Variable | Multivariate analysis | |
| OR (95%CI) | P value | |
| Time since Fontan surgery | 1.14 (1.03-1.26) | 0.010 |
| MELD-XI score | 1.40 (1.04-1.89) | 0.028 |
| Liver stiffness measurement | 1.07 (1.01-1.13) | 0.021 |
The Fontan procedure was first performed in 1968 on a patient with tricuspid atresia[14]. In the following decades, this procedure was extended to other patients with CHD and single-ventricle physiology, representing the first-line treatment for these patients. Currently, the global population of patients that have undergone Fontan surgery is estimated to exceed 70000[2]. It has led to significant improvements in survival, with an increase of > 80% within 20 years[15]. However, this surgery is associated with several long-term complications. The connection of the systemic venous return directly to the pulmonary circulation and avoiding the right heart (known as Fontan circulation) is associated with an increase in the splanchnic venous congestion and a lack of pulsability. This can lead to decreased cardiac output, resulting in ischemia[16].
Long-term effects of the Fontan circulation on the liver can cause FALD. Hemodynamic changes in the liver are associated with liver congestion and fibrosis. A study evaluating the biopsy specimens of 67 patients at ≥ 10 years since Fontan surgery revealed the presence of fibrosis in each, and a significant correlation between elapsed time since Fontan surgery and the degree of collagen deposition in the liver[5]. Although FALD has become well understood in recent years, its definition and staging varies among studies according to the criteria used, such as imaging findings or liver fibrosis. In 2023, EASL along with the ERN on rare liver diseases[6], proposed a definition of “advanced FALD” based on portal hypertension features. The diagnosis includes one or more of the following: Esophageal varices, portosystemic shunts, ascites, and/or splenomegaly.
In our study, we defined FALD based on US findings and advanced FALD using the EASL-ERN definition, and analyzed different factors associated with each one. The prevalence of FALD was approximately 90%, while the prevalence of advanced FALD was 73%. This high prevalence is presumably due to the older mean age of the patients: 33.3 ± 8.2 years, and the mean elapsed time since Fontan surgery: 24.3 ± 7.7 years, compared to other cohorts of younger-aged patients. These results support the idea that the majority of patients that have undergone Fontan surgery develop liver damage, and highlight the relevance of this disease and its complications in the overall management approach to these patients.
Several risk factors have been associated with FALD, such as elapsed time since Fontan surgery, high central venous pressure, and Fontan conduit stenosis[17-19]. Among the factors analyzed in our study, there were significant associations between FALD and elapsed time since Fontan surgery, conduit stenosis, platelet count, FIB-4, Forns index, and LSM in the univariate analysis. Statistical significance remained in multivariate analysis of the Forns index and LSM variables. Regarding advanced FALD, the univariate analysis demonstrated significant associations of advanced FALD with conduit stenosis, platelet count, GGT levels, APRI, FIB-4, Forns index, and LSM. The association between LSM and oxygen saturation was consistent in the multivariate analysis.
Noninvasive methods have limited usefulness in the treatment of FALD[20]. The Forns index, originally developed for hepatitis C virus-related hepatitis, has been evaluated in patients with FALD as the most associated serological score; However, no cut-off values have been validated[9,21]. Our data support an association between the Forns index and FALD. We also demonstrated an association between FIB-4 and FALD and advanced FALD, but a correlation with histological findings was lacking. Since FALD is not a necroinflammatory disease, rather sinusoidal congestion is the predominant mechanism of injury, scores such as APRI and FIB-4, which consider cytolysis enzymes, may not be the most appropriate. The development and validation of noninvasive scores that consider the mechanism of sinusoidal congestion are therefore needed.
TE is one of the most widely used non-invasive techniques for the assessment of liver stiffness. Several studies have evaluated the association between LSM by TE and FALD. LSM by TE was associated with elapsed time since Fontan surgery in some studies, most of which included pediatric and young patients, with a wide range of reported median values (14.6 kPa to 27.7 kPa)[22-24]. Additionally, Chemello et al[9], concluded that LSM by TE was associated with FALD (defined by imaging criteria) in 52 patients, and obtained a cut-off value for advanced FALD (defined by the presence of portal hypertension signs) of 22 kPa (AUROC, 0.930%). Few studies have compared LSM by TE and liver fibrosis in patients with FALD. There is evidence supporting the relationship between LSM and liver fibrosis; However, no cut-off points have been validated[25,26]. In 2024, based on a multicenter cohort of 217 patients, Téllez et al[11] developed the FonLiver risk score to predict severe liver fibrosis using LSM by TE and platelet count, with an AUROC of 0.810.
In our cohort of 91 patients, we demonstrated an association between LSM by TE and FALD. In addition, data from 74 patients revealed an association between LSM by TE and advanced FALD. We did not evaluate liver fibrosis because only 20 patients underwent liver biopsy, which was performed according to clinical practice. The median LSM of the total population was 25.3 kPa. In patients without FALD, median LSM was 14.6 kPa, compared to 27.7 kPa in those with FALD. These high values, even in patients without FALD, reflect the contribution of congestion to liver stiffness. These values were even higher in patients with advanced FALD, with a median LSM of 29.5 kPa compared to 19.0 kPa in those with non-advanced FALD. Since liver stiffness was associated with elapsed time since Fontan surgery, the elevated values in our cohort are probably due to the older mean age of our population and, consequently, a longer elapsed time since Fontan surgery.
We generated ROC curves for FALD and advanced FALD, and obtained AUROCs of 0.905 and 0.764, respectively. Additionally, we defined the following LSM cut-off values for FALD: Optimal cut-off value of 20 kPa (sensitivity, 92.3%; specificity, 80.0%), rule-out cut-off value of 15 kPa (sensitivity, 96.9%), and rule-in cut-off value of 25 kPa (specificity, 100%). For advanced FALD, the LSM cut-off values were: Optimal cut-off value of 25 kPa (sensitivity, 69.6%; specificity, 68.4%), rule-out cut-off value of 20 kPa (sensitivity, 95.7%), and rule-in cut-off value of 40 kPa (specificity, 94.8%). The optimal LSM cut-off values of 20 and 25 kPa for FALD and advanced FALD, respectively, were similar to those obtained in the study by Chemello et al[9], in which the optimal cut-off for FALD based on imaging findings was 22 kPa.
We developed an algorithm including elapsed time since the Fontan surgery (AUROC, 0.877; sensitivity, 95.4%; specificity, 80%; PPV, 96.9%; NPV, 72.7%; accuracy, 93.3%). Based on this algorithm, in patients with a LSM < 15 kPa, FALD can be ruled-out, whereas in patients with a LSM ≥ 25 kPa, FALD can be ruled-in. In the “grey area” of 15-25 kPa, we considered the elapsed time since Fontan surgery: When it was < 10 years, we could rule-out FALD, and when it was > 10 years, we could rule-in FALD. We attempted to combine LSM by TE with platelet count, similar to the FonLiver score. However, the AUROC was suboptimal (< 0.800).
It should be noted that in patients that have undergone Fontan surgery, LSM by TE is also influenced by liver congestion, and in this study, we could not discriminate the fibrosis component from the congestion component. It is also associated with hemodynamic variables such as pressure in the Fontan conduit and cardiac index; therefore, it may be susceptible to hemodynamics fluctuations[27]. These dynamic changes in LSM could play a role in monitoring the heart disease in these patients; however, further investigation in this setting is needed.
In other liver diseases, LSM has a demonstrated role in prognosis as revealed in 2022 by the International Consensus on Portal Hypertension Baveno VII[12]. This consensus document stated that clinically significant portal hypertension can be assumed when LSM is > 25 kPa in some etiologies (viral hepatitis, alcohol-related liver disease, and non-obese non-alcoholic steatohepatitis), and that LSM can predict the risk of liver decompensation and liver-related death. As mentioned previously, this LSM value does not apply to FALD because of the component of liver congestion. In this setting, we assessed the relationship between the LSM and prognostic variables. There was a significant association between LSM and all-cause mortality in both the univariate and multivariate analysis (OR = 1.23; 95%CI: 1.02-1.47; P = 0.026). Additionally, there was a significant association between LSM and clinically relevant events, including heart or heart-liver transplantation indication, hepatocellular carcinoma and all-cause death (OR = 1.07; 95%CI: 1.01-1.13; P = 0.03). Therefore, although we could not demonstrate the role of TE in liver fibrosis, we propose its usefulness for the prognosis of these patients.
This study has some limitations. It was a single-center observational study limited to patients in Spain, which affects the externalization of the results. Multicenter studies are therefore required for broader validation. The retrospective nature of the study and the relatively small number of patients limit its statistical power; thus, longitudinal validation in larger cohorts is also required in future studies. Furthermore, this study was based on clinical practice; therefore, all data were collected from examinations and procedures performed under medical indications. Consequently, the diagnosis of FALD was made based on imaging findings and we did not compare our results to liver biopsies. Finally, TE is limited in this setting because it cannot discriminate congestion from fibrosis, consequently LSM may be influenced by hemodynamic fluctuations.
In adult patients that have undergone Fontan surgery, TE is a useful noninvasive method for detecting FALD. In addition, the association of LSM by TE with clinically relevant events such as heart or combined heart-liver trans
The authors would like to thank all staff involved in the clinical care of patients at Hospital La Paz. The authors acknowledge Lucía S for statistical analysis.
| 1. | Kreutzer C, Kreutzer J, Kreutzer GO. Reflections on five decades of the fontan kreutzer procedure. Front Pediatr. 2013;1:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Emamaullee J, Zaidi AN, Schiano T, Kahn J, Valentino PL, Hofer RE, Taner T, Wald JW, Olthoff KM, Bucuvalas J, Fischer R. Fontan-Associated Liver Disease: Screening, Management, and Transplant Considerations. Circulation. 2020;142:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 3. | Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart. 2016;102:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 4. | Téllez L, Rodríguez-Santiago E, Albillos A. Fontan-Associated Liver Disease: A Review. Ann Hepatol. 2018;17:192-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O'Byrne ML, Lin HC, Fogel M, Rome JJ, Rand EB, Russo P, Rychik J. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Téllez L, Payancé A, Tjwa E, Del Cerro MJ, Idorn L, Ovroutski S, De Bruyne R, Verkade HJ, De Rita F, de Lange C, Angelini A, Paradis V, Rautou PE, García-Pagán JC. EASL-ERN position paper on liver involvement in patients with Fontan-type circulation. J Hepatol. 2023;79:1270-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 7. | Munsterman ID, Duijnhouwer AL, Kendall TJ, Bronkhorst CM, Ronot M, van Wettere M, van Dijk APJ, Drenth JPH, Tjwa ETTL; Nijmegen Fontan Initiative. The clinical spectrum of Fontan-associated liver disease: results from a prospective multimodality screening cohort. Eur Heart J. 2019;40:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1039] [Article Influence: 259.8] [Reference Citation Analysis (0)] |
| 9. | Chemello L, Padalino M, Zanon C, Benvegnu' L, Biffanti R, Mancuso D, Cavalletto L. Role of Transient Elastography to Stage Fontan-Associated Liver Disease (FALD) in Adults with Single Ventricle Congenital Heart Disease Correction. J Cardiovasc Dev Dis. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Yoo BW, Choi JY, Eun LY, Park HK, Park YH, Kim SU. Congestive hepatopathy after Fontan operation and related factors assessed by transient elastography. J Thorac Cardiovasc Surg. 2014;148:1498-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Téllez L, Rincón D, Payancé A, Jaillais A, Lebray P, Rodríguez de Santiago E, Clemente A, Paradis V, Lefort B, Garrido-Lestache E, Prieto R, Iserin L, Tallegas M, Garrido E, Torres M, Muriel A, Perna C, Del Cerro MJ, d'Alteroche L, Rautou PE, Bañares R, Albillos A; VALDIG-EASL consortium. Non-invasive assessment of severe liver fibrosis in patients with Fontan-associated liver disease: The VALDIG-EASL FONLIVER cohort. J Hepatol. 2025;82:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 12. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1445] [Article Influence: 481.7] [Reference Citation Analysis (2)] |
| 13. | Elder RW, McCabe NM, Hebson C, Veledar E, Romero R, Ford RM, Mahle WT, Kogon BE, Sahu A, Jokhadar M, McConnell ME, Book WM. Features of portal hypertension are associated with major adverse events in Fontan patients: the VAST study. Int J Cardiol. 2013;168:3764-3769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 1996] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 15. | Poh CL, d'Udekem Y. Life After Surviving Fontan Surgery: A Meta-Analysis of the Incidence and Predictors of Late Death. Heart Lung Circ. 2018;27:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Gewillig M. The Fontan circulation. Heart. 2005;91:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 17. | Mori M, Hebson C, Shioda K, Elder RW, Kogon BE, Rodriguez FH, Jokhadar M, Book WM. Catheter-measured Hemodynamics of Adult Fontan Circulation: Associations with Adverse Event and End-organ Dysfunctions. Congenit Heart Dis. 2016;11:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, Haw M, Cope R, Salmon AP, Sivaprakasam MC, Kendall T, Keeton BR, Iredale JP, Veldtman GR. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 289] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Camposilvan S, Milanesi O, Stellin G, Pettenazzo A, Zancan L, D'Antiga L. Liver and cardiac function in the long term after Fontan operation. Ann Thorac Surg. 2008;86:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Shin YR, Kim SU, Lee S, Choi JY, Park HK, Yoo JE, Park YN. Noninvasive surrogates are poor predictors of liver fibrosis in patients with Fontan circulation. J Thorac Cardiovasc Surg. 2022;164:1176-1185.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Baek JS, Bae EJ, Ko JS, Kim GB, Kwon BS, Lee SY, Noh CI, Park EA, Lee W. Late hepatic complications after Fontan operation; non-invasive markers of hepatic fibrosis and risk factors. Heart. 2010;96:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Schleiger A, Salzmann M, Kramer P, Danne F, Schubert S, Bassir C, Müller T, Müller HP, Berger F, Ovroutski S. Severity of Fontan-Associated Liver Disease Correlates with Fontan Hemodynamics. Pediatr Cardiol. 2020;41:736-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Fidai A, Dallaire F, Alvarez N, Balon Y, Clegg R, Connelly M, Dicke F, Fruitman D, Harder J, Myers K, Patton DJ, Prieur T, Vorhies E, Myers RP, Martin SR, Greenway SC. Non-invasive Investigations for the Diagnosis of Fontan-Associated Liver Disease in Pediatric and Adult Fontan Patients. Front Cardiovasc Med. 2017;4:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Wilson TG, d'Udekem Y, Winlaw DS, Cordina RL, Celermajer DS, Wheaton GR, Bullock A, Gentles TL, Weintraub RG, Justo RN, Grigg LE, Radford DJ, Hardikar W, Cheung M, Cain TM, Rao P, Alexander SI, Ayer J, Verrall C, Du Plessis K, Chapman J, Rice K, Barry J, Zannino D, Iyengar AJ; Australian and New Zealand Fontan Registry. Hepatic and renal end-organ damage in the Fontan circulation: A report from the Australian and New Zealand Fontan Registry. Int J Cardiol. 2018;273:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Kutty SS, Peng Q, Danford DA, Fletcher SE, Perry D, Talmon GA, Scott C, Kugler JD, Duncan KF, Quiros-Tejeira RE, Kutty S; Liver Adult-Pediatric-Congenital-Heart-Disease Dysfunction Study (LADS) Group. Increased hepatic stiffness as consequence of high hepatic afterload in the Fontan circulation: a vascular Doppler and elastography study. Hepatology. 2014;59:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Cho Y, Kabata D, Ehara E, Yamamoto A, Mizuochi T, Mushiake S, Kusano H, Kuwae Y, Suzuki T, Uchida-Kobayashi S, Morikawa H, Amano-Teranishi Y, Kioka K, Jogo A, Isoura Y, Hamazaki T, Murakami Y, Tokuhara D. Assessing liver stiffness with conventional cut-off values overestimates liver fibrosis staging in patients who received the Fontan procedure. Hepatol Res. 2021;51:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Wu FM, Opotowsky AR, Raza R, Harney S, Ukomadu C, Landzberg MJ, Valente AM, Breitbart RE, Singh MN, Gauvreau K, Jonas MM. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenit Heart Dis. 2014;9:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |