Published online Jan 7, 2025. doi: 10.3748/wjg.v31.i1.100750
Revised: October 28, 2024
Accepted: November 18, 2024
Published online: January 7, 2025
Processing time: 106 Days and 3.8 Hours
Laparoscopic liver resection (LLR) can be challenging due to the difficulty of establishing a retrohepatic tunnel under laparoscopy. Dissecting the third hepatic hilum before parenchymal transection often leads to significant liver mobilization, tumor compression, and bleeding from the short hepatic veins (SHVs). This study introduces a novel technique utilizing the ventral avascular area of the inferior vena cava (IVC), allowing SHVs to be addressed after parenchymal transection, thereby reducing surgical complexity and improving outcomes in in situ LLR.
To introduce and evaluate a novel LLR technique using the ventral avascular area of the IVC and compare its short-term outcomes with conventional methods.
The clinical cohort data of patients with pathologically confirmed hepatocellular carcinoma or intrahepatic cholangiocarcinoma who underwent conventional LLR and novel LLR between July 2021 and July 2023 at the First Affiliated Hospital of Chongqing Medical University were retrospectively analyzed. In novel LLR, we initially separated the caudate lobe from the IVC using dissecting forceps along the ventral avascular area of the IVC. Then, we transected the parenchyma of the left and right caudate lobes from the caudal side to the cephalic side using the avascular area as a marker. Subsequently, we addressed the SHVs and finally dissected the root of the right hepatic vein or left hepatic vein. The short-term postoperative outcomes and oncological results of the two approaches were evaluated and compared.
A total of 256 patients were included, with 150 (58.59%) undergoing conventional LLR and 106 (41.41%) undergoing novel LLR. The novel technique resulted in significantly larger tumor resections (6.47 ± 2.96 cm vs 4.01 ± 2.33 cm, P < 0.001), shorter operative times (199.57 ± 60.37 minutes vs 262.33 ± 83.90 minutes, P < 0.001), less intraoperative blood loss (206.92 ± 37.09 mL vs 363.34 ± 131.27 mL, P < 0.001), and greater resection volume (345.11 ± 31.40 mL vs 264.38 ± 31.98 mL, P < 0.001) compared to conventional LLR.
This novel technique enhances liver resection outcomes by reducing intraoperative complications such as bleeding and tumor compression. It facilitates a safer, in situ removal of complex liver tumors, even in challenging anato
Core Tip: This study introduces a novel laparoscopic liver resection technique utilizing the ventral avascular area of the inferior vena cava. By addressing the short hepatic veins after parenchymal transection, the technique reduces liver mobilization, tumor compression, and bleeding. Compared to conventional laparoscopic liver resection, this approach significantly decreases operative time, intraoperative blood loss, and hospital stay, while enabling safer resection of larger tumors. The method provides a valuable advancement in complex liver tumor surgeries, particularly for tumors near the inferior vena cava and third hepatic hilum.
- Citation: Huang K, Chen Z, Xiao H, Hu HY, Chen XY, Du CY, Lan X. Laparoscopic liver resection utilizing the ventral avascular area of the inferior vena cava: A retrospective cohort study. World J Gastroenterol 2025; 31(1): 100750
- URL: https://www.wjgnet.com/1007-9327/full/v31/i1/100750.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i1.100750
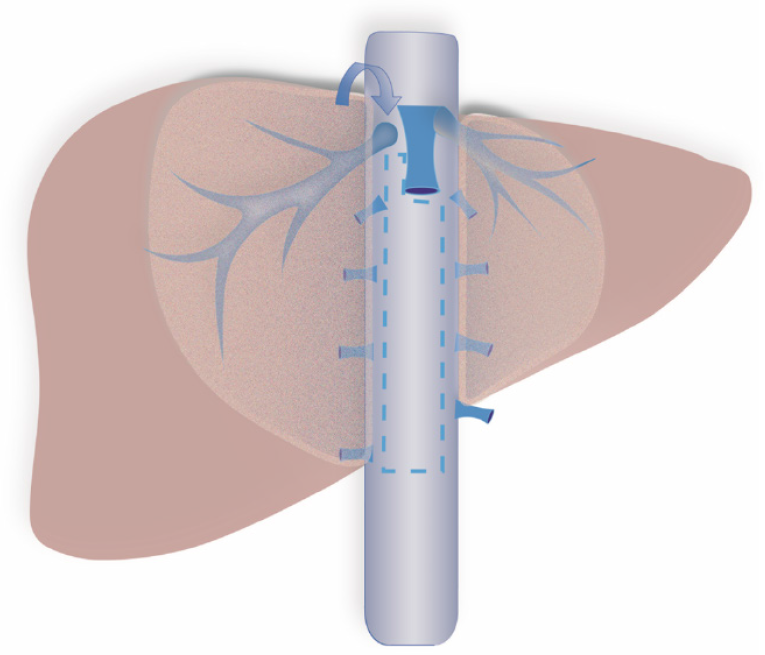
On the ventral surface of the retrohepatic inferior vena cava (IVC), there is an avascular space with a width of 1 cm (Figure 1)[1]. In open liver resection, surgeons can easily implement the liver hanging maneuver by utilizing this avascular space to establish a retrohepatic tunnel (RT), thereby avoiding liver mobilization, tumor compression, and injury of the IVC, ultimately achieving in situ resection of liver tumors[1]. However, the limited anatomical space in laparoscopic liver resection (LLR) makes it difficult to establish this RT under laparoscopy. As a result, surgeons traditionally prioritize dissecting the short hepatic veins (SHVs) at the third hepatic hilum during procedures such as right hemihepatectomy, right posterior lobectomy, and segment VII resection[2,3]. While this approach can improve surgical visualization and facilitate liver mobilization, it carries several risks. Unexpected bleeding can occur during SHV dissection due to their proximity to major vasculature. Additionally, rotation of the hepatoduodenal ligament during this process can lead to ischemia of the left hepatic lobe, and tumor compression increases the potential for hematogenous dissemination of cancer cells[4]. The risks are particularly pronounced when tumors are located near the IVC or invade the adrenal gland. In these cases, addressing the third hepatic hilum prior to liver parenchymal transection can result in iatrogenic tumor rupture or severe bleeding due to accidental IVC injury. Furthermore, the challenging anatomical position may make it infeasible to address the third hepatic hilum before the liver parenchymal transection. In this study, we first report a novel surgical technique utilizing this avascular area to establish an RT more easily under laparoscopy, optimizing the surgical process in the relevant area, reducing surgical difficulty, and enabling certain procedures that previously needed laparotomy to be performed laparoscopically.
Patients with pathologically confirmed hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma (ICC) who underwent conventional LLR and novel LLR between July 2021 and July 2023 at the First Affiliated Hospital of Chongqing Medical University were studied retrospectively. Clinical data (age, sex, liver function before and after the operation, complications, hospital stay after the operation, operative time, hemorrhage, ascites, and perioperative mortality) were collected. Patients with intrahepatic or extrahepatic metastases; those who underwent partial non-anatomical resections, and those with incomplete clinical data were excluded. All patients provided written informed consent for surgery, and this study was approved by the ethical review board of the First Affiliated Hospital of Chongqing Medical University.
In this study, we retrospectively analyzed patients with pathologically confirmed HCC or ICC who underwent LLR between July 2021 and July 2023. The exclusion criteria were as follows: (1) Patients with intrahepatic or extrahepatic metastases, as such cases may require different surgical approaches and could confound the results of this study; (2) Patients who underwent partial non-anatomical resections, as this type of surgery does not align with the scope of our comparison between conventional and novel LLR techniques; and (3) Patients with incomplete clinical data, which would have made it difficult to ensure reliable outcome measures.
The details of the surgical procedures were described in our previous study[5]. This novel technique involves three steps: (1) Initially, the caudate lobe is separated from the IVC using dissecting forceps along the ventral avascular area of the IVC; (2) Then the parenchyma of the left and right caudate lobes is transected from the caudal side to the cephalic side using the avascular area as a marker; and (3) The SHVs are subsequently addressed, and the root of the right hepatic vein (RHV) or left hepatic vein is finally dissected. To fully illustrate the concrete steps of each surgical procedure, we have provided corresponding surgical short videos and surgical diagrams for each surgical approach. Taking right hemihepatectomy as an example, we initially occluded and resected the right liver pedicles to better demarcate the resection boundary of the liver. Then, the extent of resection was determined based on the extent of hepatic ischemia. We first transected the liver parenchyma along the ischemic demarcation line, middle hepatic vein (MHV) and IVC before addressing the right liver ligament and SHVs, transected the liver parenchyma along the ischemic demarcation line, MHV and IVC, and then addressed the right liver ligament and SHVs. In approaching the IVC, we separated the caudate lobe from the IVC using dissecting forceps along the ventral avascular region. Then, the above steps were repeated until the whole ventral side of the retrohepatic IVC was exposed. Next, the SHVs, the Makuuchi ligament, and the root of the RHV were dissected along a caudal-to-cranial direction until the conclusion of the entire hepatectomy procedure.
The data processing and analysis were conducted using the R software (version 4.3.3, R Foundation for Statistical Computing). Prior to statistical evaluation, all datasets underwent standard normality assessments with the Shapiro-Wilk test. Continuous data that were normally distributed are reported as mean ± SD. For comparing two distinct groups, a two-sample t-test was applied. Should the data fail to meet normal distribution criteria, the median and interquartile range [M (P25, P75)] were reported, and the Mann-Whitney U-test, a nonparametric method, was utilized for statistical analysis. Categorical data were described using the number of cases and percentage. For intergroup comparisons of categorical variables, the χ2 test was employed, with Fisher’s exact test serving as an alternative when χ2 test assumptions were not satisfied. All statistical tests were two-tailed, and P value of less than 0.05 was considered indicative of statistical significance.
To better demonstrate the advantages of our novel technique, we first report a case utilizing the conventional surgical approach, leading to intraoperative hemorrhage. A 50-year-old male patient diagnosed with HCC underwent laparoscopic segment VII resection. As usual, we prioritized dissecting the right ligaments of the liver and the SHVs of the third hepatic hilum. The objective of these steps was to achieve complete mobilization of the right lobe and obtain a better surgical vision. Unfortunately, during the process of dissecting the SHVs, there was significant unexpected hemorrhage because of the removal of the SHVs. However, due to the limited anatomical space caused by this conventional approach, controlling the bleeding was very difficult (Figure 2, Video 1).
LLR for segment 7 and 8: A 50-year-old male patient was initially diagnosed with segment 7 and the dorsal side of segment 8-located HCC with a diameter of 5 cm × 4 cm (Figure 3A and B). We can see that the hepatic pedicle of S8 dorsal is located on the dorsal side and left of the right anterior hepatic pedicle (Figure 3C). After clamping the S8 dorsal branches and the S7 pedicles, we can clearly see the dorsal and ventral boundaries of segment 8 and the boundaries of segments 6 and 7 (Figure 3D and E). We do not dissect the third hepatic portal before we severed the liver parenchyma, and the parenchyma is transected along the stain or ischemic demarcation line (Figure 3F). After we transect the G7 and dorsal side of G8, liver parenchyma is transected along the ventral avascular area of the IVC (Figure 3G-I). And then, we can easily address the SHV, RHV, and Makuuchi ligament (Figure 3J and K, Video 2).
LLR for segment 7: A 66-year-old female patient was initially diagnosed with segment 7-located HCC with a diameter of 3 cm × 4 cm (Figure 4A and B). The anatomy of the Laennec membrane starts from the dorsal side of the posterior pedicle and we dissect the G7 and then clamp it (Figure 4C). And then, the extent of resection was determined based on the extent of hepatic ischemia. To better mark the surgical resection boundary, we utilized the technique of fluorescent reverse staining (Figure 4D). We do not dissect the third hepatic portal before we severed the liver parenchyma, and the liver parenchyma is transected along the indocyanine green staining demarcation line and RHV (Figure 4E). After we transect the G7, liver parenchyma is transected along the ventral avascular area of the IVC (Figure 4F and G). And then, we can easily address the SHV, Makuuchi ligament and right posterior inferior vein (Figure 4H-K, Video 3).
Right hemihepatectomy for tumors invading the right adrenal gland and IVC: A 57-year-old male patient was initially diagnosed with right liver lobe-located HCC with a diameter of 7.5 cm × 6.1 cm. The tumor invaded the right adrenal gland and IVC, as shown by computed tomography and 3-dimensional reconstruction (Figure 5A and B). After the multidisciplinary team discussion, we performed a laparoscopic right hepatectomy on the patient. Were traditional laparoscopic right hepatectomies performed, the first step would be to mobilize the liver and address the third hepatic hilum. However, in this specific patient’s case, because of the tumor’s adjacency to the third hepatic hilum and its invasion of the IVC, the liver mobilization process could not be performed. Additionally, during the process, there was a high risk of tumor rupture and injury to the IVC, leading to significant intraoperative bleeding. In this situation, we successfully employed our novel technique to establish a tunnel by utilizing the ventral avascular area of the retrohepatic IVC, and this innovative approach significantly reduced the surgical difficulty and risk. Following the aforementioned steps, we first transected the liver parenchyma along the ischemic demarcation line, MHV and IVC before addressing the right liver ligament and SHVs. While approaching the IVC, we separated the caudate lobe from the IVC along the ventral avascular region and transected the caudate lobe along this tunnel after RT was established (Figure 5C and D). We repeated the above steps until the whole ventral side of the retrohepatic IVC was exposed (Figure 5E and F). We dissected the SHVs and the root of the RHV (Figure 5G-I). Subsequently, we proceeded with the excision of the tumor and the involved right adrenal gland and IVC and with the repair of the IVC (Figure 5J). Ultimately, the Makuuchi ligament was meticulously dissected, signifying the conclusion of the entire hepatectomy procedure (Figure 5K, Video 4). During the surgical procedure, we discovered that this novel surgical procedure provided a sufficient surgical field for tumor removal and facilitated repair of the IVC.
Posterior and anterodorsal segment resection: A 50-year-old male patient was initially diagnosed with a right posterior and anterodorsal lobe-located HCC with a diameter of 5.9 cm × 6.8 cm (Figure 6A). The patient underwent laparoscopic posterior and anterodorsal segment resection (Figure 6B). To better demarcate the resection boundary of the liver in this patient, we utilized the technique of fluorescent reverse staining. We initially occluded and resected the target liver pedicles (posterior pedicle and antero-dorsal pedicles) (Figure 6C). Following the aforementioned method, we also first transected the liver parenchyma along the fluorescence staining boundary, the anterior fissure vein, and IVC before addressing the right liver ligament and the SHVs. When approaching the IVC, we separated the caudate lobe from the IVC using dissecting forceps along the ventral avascular region (Figure 6D). After the RT was established, we transected the caudate lobe along this tunnel (Figure 6E) and then repeated the above steps until the whole ventral side of the retrohepatic IVC was exposed (Figure 6F-J). After that, the SHVs and the Makuuchi ligament were dissected from the caudate side to the cephalic side (Figure 6K-N, Video 5).
Posterior segment resection: A 52-year-old male patient was initially diagnosed with a right posterior lobe-located HCC with a diameter of 3.5 cm × 3.2 cm (Figure 7A). The patient underwent resection of the right posterior lobe of the liver (Figure 7B). After completing the occlusion of the right posterior hepatic pedicle, the extent of resection was determined based on the extent of hepatic ischemia (Figure 7C). Similarly, we first transected the liver parenchyma along the ischemic demarcation line, RHV and IVC, and then addressed the right liver ligament and the SHVs. While approaching the IVC, we also separated the caudate lobe from the IVC using dissecting forceps along the ventral avascular region and then repeated the above steps until the whole ventral side of the retrohepatic IVC was exposed (Figure 7D-J). Next, the SHVs and the Makuuchi ligament were dissected along a caudal-to-cranial direction (Figure 7K and L, Video 6).
Left hemihepatectomy: An 80-year-old female patient was initially diagnosed with a left lobe-located ICC with a dia
Right hemihepatectomy: A 47-year-old male patient was initially diagnosed with HCC with a diameter of 5.8 cm × 6.6 cm located in the right lobe (Figure 9A). Due to the tumor being in proximity to the right hepatic pedicle, laparoscopic right hemihepatectomy was considered (Figure 9B). Before addressing the right liver ligament and the SHVs, we first transected the liver parenchyma along the ischemic demarcation line, MHV and IVC. When approaching the IVC, the caudate lobe was separated from the IVC using dissecting forceps along the ventral avascular area (Figure 9C). After the RT was established, we transected the caudate lobe along this tunnel (Figure 9D). Then, we repeated the above steps until the whole ventral side of the retrohepatic IVC was exposed (Figure 9E-G). After that, the SHVs, right adrenal gland, root of the RHV, Makuuchi ligament, and right liver ligament were dissected from the caudate side to the cephalic side (Figure 9H-K, Video 8).
Baseline clinical features: In this study, a total of 256 patients were included, with 150 in the conventional LLR group and 106 in the novel LLR group. Among the 256 patients, there were 130 males (50.8%) and 126 females (49.2%), and the pathological types included HCC in 176 patients (68.8%) and ICC in 80 patients (31.2%). Cirrhosis was present in 157 patients (61.3%). The Barcelona Clinic Liver Cancer staging was predominantly in stage A, accounting for 230 patients (89.8%). In the Chinese liver cancer staging, stage 1A was the main classification, occurring in 207 patients (80.9%), and 249 patients (97.3%) had a Child-Pugh grade of A. The clinical characteristics of the patients are summarized in Table 1. Except for the Barcelona Clinic Liver Cancer staging and preoperative indocyanine green retention rate, there were no statistically significant differences between the two groups (Table 1).
| Overall (n = 256) | Conventional LLR (n = 150) | Novel LLR (n = 106) | P value | |
| Age, year | 62.88 ± 10.52 | 62.43 ± 11.07 | 63.52 ± 9.71 | 0.414 |
| Sex | 1.000 | |||
| Male | 130 (50.8) | 76 (50.7) | 54 (50.9) | |
| Female | 126 (49.2) | 74 (49.3) | 52 (49.1) | |
| Pathological types | 0.707 | |||
| HCC | 176 (68.8) | 105 (70.0) | 71 (67.0) | |
| ICC | 80 (31.2) | 45 (30.0) | 35 (33.0) | |
| BMI, kg/m2 | 23.77 ± 4.30 | 23.90 ± 4.29 | 23.59 ± 4.33 | 0.567 |
| Hepatitis B/C virus | 0.119 | |||
| B | 212 (82.8) | 130 (86.7) | 82 (77.4) | |
| C | 9 (3.5) | 5 (3.3) | 4 (3.8) | |
| None | 35 (13.7) | 15 (10.0) | 20 (18.9) | |
| Cirrhosis | 0.694 | |||
| No | 99 (38.7) | 56 (37.3) | 43 (40.6) | |
| Yes | 157 (61.3) | 94 (62.7) | 63 (59.4) | |
| Comorbidities | 0.729 | |||
| No | 118 (46.1) | 71 (47.3) | 47 (44.3) | |
| Yes | 138 (53.9) | 79 (52.7) | 59 (55.7) | |
| BCLC | < 0.001 | |||
| 0 | 22 (8.6) | 21 (14.0) | 1 (0.9) | |
| A | 230 (89.8) | 129 (86.0) | 101 (95.3) | |
| B | 2 (0.8) | 0 (0.0) | 2 (1.9) | |
| C | 2 (0.8) | 0 (0.0) | 2 (1.9) | |
| CNLC | 0.650 | |||
| 1A | 207 (80.9) | 119 (79.3) | 88 (83.0) | |
| 1B | 45 (17.6) | 29 (19.3) | 16 (15.1) | |
| 2A | 4 (1.6) | 2 (1.3) | 2 (1.9) | |
| ECOG | 0.222 | |||
| 0 | 247 (96.5) | 147 (98.0) | 100 (94.3) | |
| 1 | 9 (3.5) | 3 (2.0) | 6 (5.7) | |
| Child-Pugh grade | 0.757 | |||
| A | 249 (97.3) | 145 (96.7) | 104 (98.1) | |
| B | 7 (2.7) | 5 (3.3) | 2 (1.9) | |
| Preoperative ICG retention rate | 5.00 ± 2.06 | 3.57 ± 1.18 | 7.01 ± 1.14 | < 0.001 |
| Conversion therapy | 1.000 | |||
| No | 253 (98.8) | 148 (98.7) | 105 (99.1) | |
| Yes | 3 (1.2) | 2 (1.3) | 1 (0.9) |
Intraoperative states: Intraoperative data are summarized (Table 2). All 256 patients underwent R0 resection. The tumor size (cm), the operative time (minute), and the minimum width of the surgical margin (mm) were 5.03 ± 2.88, 236.34 ± 81.08, and 18.95 ± 6.33, respectively. Compared with conventional LLR, novel LLR was associated with larger tumor sizes (6.47 ± 2.96 cm vs 4.01 ± 2.33 cm, P < 0.001), shorter operative times (199.57 ± 60.37 minutes vs 262.33 ± 83.90 minutes, P < 0.001), less blood loss (206.92 ± 37.09 mL vs 363.34 ± 131.27 mL, P < 0.001), and a greater preoperative resection volume (345.11 ± 31.40 mL vs 264.38 ± 31.98 mL, P < 0.001).
| Overall (n = 256) | Conventional LLR (n = 150) | Novel LLR (n = 106) | P value | |
| Tumor size, cm | 5.03 ± 2.88 | 4.01 ± 2.33 | 6.47 ± 2.96 | < 0.001 |
| Tumor location | 31 (12.1) | 15 (10.0) | 16 (15.1) | 0.218 |
| S II | 23 (9.0) | 12 (8.0) | 11 (10.4) | |
| S III | 39 (15.2) | 27 (18.0) | 12 (11.3) | |
| S IV | 46 (18.0) | 32 (21.3) | 14 (13.2) | |
| S V | 37 (14.5) | 19 (12.7) | 18 (17.0) | |
| S VI | 33 (12.9) | 18 (12.0) | 15 (14.2) | |
| S VII | 36 (14.1) | 22 (14.7) | 14 (13.2) | |
| S VIII | 2 (0.8) | 2 (1.3) | 0 (0.0) | |
| S II/III | 9 (3.5) | 3 (2.0) | 6 (5.7) | |
| Type of resection | 48 (18.8) | 36 (24.0) | 12 (11.3) | 0.051 |
| S5 + S6 + S7 + S8 | 43 (16.8) | 25 (16.7) | 18 (17.0) | |
| S2 + S3 + S4 | 38 (14.8) | 22 (14.7) | 16 (15.1) | |
| S6 + S7 | 35 (13.7) | 23 (15.3) | 12 (11.3) | |
| S6 + S7 + S5v + S8v | 38 (14.8) | 16 (10.7) | 22 (20.8) | |
| S7 | 27 (10.5) | 16 (10.7) | 11 (10.4) | |
| S7 + S8 | 27 (10.5) | 12 (8.0) | 15 (14.2) | |
| Operative time, minute | 236.34 ± 81.08 | 262.33 ± 83.90 | 199.57 ± 60.37 | < 0.001 |
| Blood loss | 298.57 ± 128.82 | 363.34 ± 131.27 | 206.92 ± 37.09 | < 0.001 |
| Number of lesions | 0.165 | |||
| 1 | 251 (98.0) | 145 (96.7) | 106 (100.0) | |
| 2 | 3 (1.2) | 3 (2.0) | 0 (0.0) | |
| 3 | 2 (0.8) | 2 (1.3) | 0 (0.0) | |
| Distance from major vessels | 0.944 | |||
| > 1 cm | 189 (73.8) | 110 (73.3) | 79 (74.5) | |
| < 1 cm | 67 (26.2) | 40 (26.7) | 27 (25.5) | |
| Distance from liver capsule | 0.899 | |||
| < 1 cm | 128 (50.0) | 76 (50.7) | 52 (49.1) | |
| > 1 cm | 128 (50.0) | 74 (49.3) | 54 (50.9) | |
| Preoperative resection volume, mL | 297.81 ± 50.90 | 264.38 ± 31.98 | 345.11 ± 31.40 | < 0.001 |
| PRLV/TLV | 30.58 ± 10.06 | 30.37 ± 10.21 | 30.87 ± 9.89 | 0.697 |
| Minimum surgical margin width, mm | 18.95 ± 6.33 | 18.96 ± 6.27 | 18.94 ± 6.45 | 0.984 |
| Total pringle maneuver time, minute | 44.93 ± 10.53 | 45.12 ± 10.49 | 44.67 ± 10.64 | 0.737 |
| ICG staining method | 0.887 | |||
| None | 176 (68.8) | 104 (69.3) | 72 (67.9) | |
| Negative staining | 52 (20.3) | 29 (19.3) | 23 (21.7) | |
| Positive staining | 28 (10.9) | 17 (11.3) | 11 (10.4) | |
| Blood transfusion | 0.761 | |||
| No | 253 (98.8) | 149 (99.3) | 104 (98.1) | |
| Yes | 3 (1.2) | 1 (0.7) | 2 (1.9) | |
| R0 resection Y | 256 (100.0) | 150 (100.0) | 106 (100.0) | NA |
Postoperative complications and findings during follow up: Postoperative data are summarized (Table 3). All patients recovered uneventfully after surgery. Among the 256 patients, 254 (99.2%) had a Clavien-Dindo complication grade of < IIIa. There were no deaths within 90 days postoperatively. The mean follow-up duration was 10 months, with 1 (0.4%) patient experiencing readmission, 2 (0.8%) patients having a recurrence, 1 (0.4%) patient developing liver failure, and 3 (1.2%) patients suffering from ascites. In comparison with conventional LLR, novel LLR was associated with a shorter postoperative hospital stay (4.59 ± 1.02 days vs 5.90 ± 2.17 days, P < 0.001).
| Overall (n = 256) | Conventional LLR (n = 150) | Novel LLR (n = 106) | P value | |
| 90 days mortality | ||||
| No | 256 (100.0) | 150 (100.0) | 106 (100.0) | |
| Postoperative hospital stays, day | 5.36 ± 1.89 | 5.90 ± 2.17 | 4.59 ± 1.02 | < 0.001 |
| Readmission | 1.000 | |||
| No | 255 (99.6) | 149 (99.3) | 106 (100.0) | |
| Yes | 1 (0.4) | 1 (0.7) | 0 (0.0) | |
| Recurrence | 0.636 | |||
| No | 254 (99.2) | 148 (98.7) | 106 (100.0) | |
| Yes | 2 (0.8) | 2 (1.3) | 0 (0.0) | |
| Follow up months | 10 (5, 15) | 10 (5, 15) | 11 (5, 16) | 0.346 |
| Liver failure | 1.000 | |||
| No | 255 (99.6) | 149 (99.3) | 106 (100.0) | |
| Yes | 1 (0.4) | 1 (0.7) | 0 (0.0) | |
| Ascites | 0.382 | |||
| No | 253 (98.8) | 147 (98.0) | 106 (100.0) | |
| Yes | 3 (1.2) | 3 (2.0) | 0 (0.0) | |
| Clavien-Dindo complication grade | 0.636 | |||
| < IIIa | 254 (99.2) | 148 (98.7) | 106 (100.0) | |
| ≥ IIIa | 2 (0.8) | 2 (1.3) | 0 (0.0) |
Although LLR is currently recognized as a standard surgical technique and has developed into the preferred approach for HCC and ICC[6-8], intraoperative bleeding is the main problem in hepatectomy. Blood loss, which is both a risk factor and a predictive factor, is associated with perioperative morbidity and mortality[9], and is associated with recurrence and survival after resection of HCC[10]. Therefore, bleeding control is important during liver resection, especially for patients with cirrhosis[7,11]. In conventional laparoscopic hepatectomy, complete mobilization of the right liver is performed before liver parenchyma transection, which could increase the risk of excessive bleeding from the right liver attachments, iatrogenic tumor rupture, left hepatic lobe ischemia from the rotation of the hepatoduodenal ligament, and hematogenous tumor cell dissemination[4]. As illustrated in case 1, we performed a conventional LLR for the resection of segment 7. During the process of dissecting the SHVs, unexpected and significant hemorrhage occurred. Controlling the bleeding was very difficult due to the poor visibility in the surgical field.
To address these issues, it is advisable to opt for the anterior approach or in situ resection, wherein liver mobilization is executed after parenchymal transection[12]. In subsequent studies, it has also been demonstrated that this technique provides improved operative and survival outcomes[13-15]. To achieve in situ resection of liver tumors, Belghiti et al[1] designed a liver hanging maneuver for open liver resection in 2001 by inserting a tape between the anterior surface of the vena cava and the liver. This technique was subsequently confirmed to have many advantages, such as better control of bleeding[16], but it is still challenging to establish an RT to perform the liver hanging maneuver in LLR. The main reasons can be attributed to the restrictions imposed by the caudal view and the constraints of the anatomical space. Therefore, surgeons still typically prioritize dissecting the SHVs of the third hepatic hilum. However, in addition to the disad
In contrast to the aforementioned methods, in our study, we designed a novel technique to achieve in situ resection for LLR. Although the novel technique was implemented in a high-experience center, it has significant potential for broader adoption in centers with limited experience in complex LLR procedures. The technique had advantages in simplifying the surgical procedure, preventing liver rotation and intraoperative bleeding caused by prior management of the SHV, improving the visibility of the surgical field, reducing the risk of tumor rupture, and establishing a well-defined navigational plane based on the IVC. Most importantly, we believe that the novel technique will be an optimal approach for tumors involving the third hepatic hilum or the IVC, allowing for addressing more complex intraoperative situations. Simultaneously, this method is easily learnable and replicable, making it suitable for surgical doctors who are just beginning to perform LLRs and who need to handle complex situations during the procedure. By using the avascular area of the IVC as a clear anatomical landmark, this technique reduces the need for extensive liver mobilization and minimizes intraoperative bleeding risks. These features simplify the learning curve, making the technique safer and more feasible for centers without extensive experience in advanced laparoscopic techniques.
Performing LLR for lesions in the posterosuperior segments is more technically demanding. In our study, we observed that utilizing the novel technique for resecting segments 6 and/or 7 reduced the technical difficulty of the surgery, improved surgical visibility, decreased the risk of intraoperative bleeding, and decreased the risk of excessive bleeding from the right liver attachments, iatrogenic tumor rupture, and left hepatic lobe ischemia from rotation of the hepatoduodenal ligament. Moreover, it maximized the benefits of the caudal view in LLR. In addition, we applied this technique in various types of LLR, including right hemihepatectomy, posterior and anterodorsal segment resection, posterior segment resection, and hemihepatectomy, and observed consistent advantages. Another advantage of our technique is that it establishes a well-defined navigational plane based on the IVC. As we well know, cirrhosis is the strongest risk factor for HCC[19]. In Eastern Asia, where most HCC patients have liver cirrhosis due to chronic viral infections, surgeons face the challenge of achieving both oncologic ability and preserving liver parenchyma during liver surgery. By relying on these landmarks, surgeons can avoid entering incorrect planes and ensure the precision needed for LLR.
Most importantly, this novel technique may be an optimal approach for tumors invading the IVC and the adrenal gland. In our study, when traditional laparoscopic right hepatectomy was performed, the first step was to mobilize the liver and address the third hepatic hilum (Figure 7). However, in this specific patient’s case, the liver mobilization process could not be performed due to the tumor’s adjacency to the third hepatic hilum and its invasion of the IVC. Additionally, during the process, there is a high risk of tumor rupture and injury to the IVC, leading to significant intraoperative bleeding. In this situation, we successfully employed our novel technique to establish a tunnel by utilizing the ventral avascular area of the retrohepatic IVC, and this innovative approach significantly reduced the surgical difficulty and risk.
Robotic-assisted liver resection (RALR) is another emerging approach[20-22], valued for its enhanced precision, dexterity, and 3-dimensional visualization. However, RALR is associated with high costs and requires specialized equipment, limiting its accessibility. In contrast, our novel laparoscopic technique offers a cost-effective alternative, providing similar benefits by using the IVC avascular area as a guiding landmark to avoid extensive liver mobilization and improve accuracy. While RALR may offer additional maneuverability in specific cases, our technique presents a simpler, accessible approach that can achieve comparable in situ resection outcomes without the significant resource demands.
To our knowledge, this is the first study to introduce the LLR approach utilizing the ventral avascular area of the IVC. At our medical center, all procedures involving the specified region are conducted in this surgical approach. Our observation is that the utilization of this method markedly diminishes the operative complexity and risk. Additionally, it substantially reduces the conversion rate in cases where tumors infiltrate the IVC, and we have not encountered any cases that required a conversion to laparotomy. There remain some limitations to this novel approach. First, due to the retrospective nature of this study, selection bias is unavoidable, which may impact the generalizability of the findings. Second, although this technique significantly simplifies certain aspects of LLR, it still requires a foundational proficiency in laparoscopic surgery, potentially presenting a learning curve challenge for centers without experience in complex liver resections. Third, our study primarily addresses short-term outcomes, and long-term survival and recurrence data are needed to fully understand the oncological efficacy of this approach. Additionally, patient selection remains an important consideration. This technique may be less suitable for cases involving extensive IVC invasion, where open surgery might be necessary for complete tumor resection. Finally, the relatively small sample size of our study limits the robustness of our findings, underscoring the need for large-scale, multicenter clinical trials to further evaluate the safety and efficacy of this approach. Moreover, while this technique is more cost-effective compared to RALR, it requires specialized training in laparoscopic techniques. Centers interested in implementing this approach should consider providing adequate resources and training programs to ensure safe and effective adoption.
This study demonstrates that using the ventral avascular area of the IVC in LLR offers key advantages, including reduced bleeding, minimized liver mobilization, and improved visibility, making it valuable for complex resections near the IVC and third hepatic hilum. These positive outcomes suggest this technique could shape future guidelines by providing a safer, standardized approach, adaptable across centers with varied experience levels in LLR. Further multicenter studies are needed to validate these findings, potentially advancing this method as a best practice and improving outcomes in complex liver resections.
| 1. | Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 356] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Liu F, Zhang J, Lei C, Wei Y, Li B. Feasibility of laparoscopic major hepatectomy for hepatic paragonimiasis: two case reports. Medicine (Baltimore). 2016;95:e4939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Li H, Wei Y, Li B, Peng B. The First Case of Total Laparoscopic Living Donor Right Hemihepatectomy in Mainland China and Literature Review. Surg Laparosc Endosc Percutan Tech. 2016;26:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Zhou YM, Sui CJ, Zhang XF, Li B, Yang JM. Anterior approach combined with infrahepatic inferior vena cava clamping right hepatic resection for large hepatocellular carcinoma: A prospective randomized controlled trial. Medicine (Baltimore). 2016;95:e4159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Lan X, Zhang HL, Zhang H, Peng YF, Liu F, Li B, Wei YG. Four-year experience with more than 1000 cases of total laparoscopic liver resection in a single center. World J Gastroenterol. 2022;28:2968-2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Araki K, Fuks D, Nomi T, Ogiso S, Lozano RR, Kuwano H, Gayet B. Feasibility of laparoscopic liver resection for caudate lobe: technical strategy and comparative analysis with anteroinferior and posterosuperior segments. Surg Endosc. 2016;30:4300-4306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Lan X, Li H, Liu F, Li B, Wei Y, Zhang H, Xu H. Does liver cirrhosis have an impact on the results of different hepatic inflow occlusion methods in laparoscopic liver resection? a propensity score analysis. HPB (Oxford). 2019;21:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Wakabayashi G, Cherqui D, Geller DA, Han HS, Kaneko H, Buell JF. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2014;21:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406. [PubMed] |
| 10. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D'Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 11. | Yoon YS, Han HS, Cho JY, Ahn KS. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc. 2010;24:1630-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Lin TY, Sridharan M, Ho ST. Retrograde resection of hepatic lobe for extensive carcinoma of the liver. Med Chir Dig. 1977;6:87-88. [PubMed] |
| 13. | Lai EC, Fan ST, Lo CM, Chu KM, Liu CL. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314-7; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Li SQ, Liang LJ, Peng BG, Yin XY, Lü MD, Kuang M, Li DM, Fu SJ. [A comparative study of anterior versus conventional approach right hepatectomy for large hepatocellular carcinoma]. Zhonghua Yi Xue Za Zhi. 2010;90:1670-1673. [PubMed] |
| 16. | Wang CC, Jawade K, Yap AQ, Concejero AM, Lin CY, Chen CL. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World J Surg. 2010;34:1874-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Chu H, Cao G, Tang Y, Du X, Min X, Wan C. Laparoscopic liver hanging maneuver through the retrohepatic tunnel on the right side of the inferior vena cava combined with a simple vascular occlusion technique for laparoscopic right hemihepatectomy. Surg Endosc. 2018;32:2932-2938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Cai LX, Wei FQ, Yu YC, Cai XJ. Can retrohepatic tunnel be quickly and easily established for laparoscopic liver hanging maneuver by Goldfinger dissector in laparoscopic right hepatectomy? J Zhejiang Univ Sci B. 2016;17:712-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Park S, Davis AM, Pillai AA. Prevention, Diagnosis, and Treatment of Hepatocellular Carcinoma. JAMA. 2024;332:1013-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Levi Sandri GB, de Werra E, Mascianà G, Colasanti M, Santoro R, D'Andrea V, Ettorre GM. Laparoscopic and robotic approach for hepatocellular carcinoma-state of the art. Hepatobiliary Surg Nutr. 2016;5:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Safiejko K, Pedziwiatr M, Pruc M, Tarkowski R, Juchimiuk M, Domurat M, Smereka J, Anvarov K, Sielicki P, Kurek K, Szarpak L. Robotic versus Laparoscopic Liver Resections for Colorectal Metastases: A Systematic Review and Meta-Analysis. Cancers (Basel). 2024;16:1596. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Liu J, Dou C, Chen J, Lu Y, Liang L, Wei F, Zhang C. Evaluation of the outcomes of biliary-enteric reconstruction in robotic radical resection of hilar cholangiocarcinoma: a single-center propensity score matching analysis. Sci Rep. 2024;14:14836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |