Published online Feb 21, 2024. doi: 10.3748/wjg.v30.i7.759
Peer-review started: November 19, 2023
First decision: December 15, 2023
Revised: December 18, 2023
Accepted: January 23, 2024
Article in press: January 23, 2024
Published online: February 21, 2024
Processing time: 93 Days and 20.8 Hours
Most patients with advanced pancreatic neuroendocrine tumors (pNETs) die due to tumor progression. Therefore, identifying new therapies with low toxicity and good tolerability to use concomitantly with the established pNET treatment is relevant. In this perspective, metformin is emerging as a molecule of interest. Retrospective studies have described metformin, a widely used agent for the treatment of patients with type 2 diabetes mellitus (T2DM), to be effective in modulating different tumor-related events, including cancer incidence, recurrence and survival by inhibiting mTOR phosphorylation. This systematic review eva
To systematically analyze and summarize evidence related to the diagnostic and prognostic value of T2DM and metformin for predicting the insurgence and post-treatment outcomes of pNET.
A systematic review of the published literature was undertaken, focusing on the role of T2DM and metformin in insurgence and prognosis of pNET, measured through outcomes of tumor-free survival (TFS), overall survival and progression-free survival.
A total of 13 studies (5674 patients) were included in this review. Analysis of 809 pNET cases from five retro
T2DM represents a risk factor for the insurgence of pNET and is a significant predictor of poor post-treatment TFS of pNET patients. Unfortunately, a few studies with heterogeneous results limited the possibility of exploring the effect of metformin in the diagnosis and prognosis of pNET.
Core Tip: Pancreatic neuroendocrine tumors (pNETs) are a challenge to diagnose and treat. Often curative treatments are not possible and additional therapy aimed at symptom relief and tumor cell growth inhibition is warranted. Unfortunately, a significant number of pNET patients do not respond to the above-mentioned medical treatments or show resistance. Therefore, exploring the risk factors and additional therapeutics is of importance. This systematic review and meta-analysis showed that in patients with type 2 diabetes mellitus (T2DM), the risk for pNET insurgence was significantly increased. In addition, T2DM was a significant predictor of poor tumor free survival. Results on the role of metformin in the setting of diagnosis and prognosis of pNET due to paucity of data and data heterogeneity failed to show statistical relevance of its use, although there are indices that it might positively impact the progression free survival.
- Citation: Cigrovski Berkovic M, Coppola A, Sesa V, Mrzljak A, Lai Q. Metformin and pancreatic neuroendocrine tumors: A systematic review and meta-analysis. World J Gastroenterol 2024; 30(7): 759-769
- URL: https://www.wjgnet.com/1007-9327/full/v30/i7/759.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i7.759
The incidence of pancreatic neuroendocrine tumors (pNETs), especially non-functioning ones, increased more than 3-fold in the last few decades. Moreover, due to their relatively indolent course, the prevalence of pNETs is also rising, so they account for approximately 10% of all pancreatic neoplasms[1]. Unfortunately, due to an asymptomatic course, diagnosis is still challenging, and tumors are often diagnosed at the metastatic stage, with spread mainly to the liver[2,3], when curative treatments, such as surgical removal of primary tumors, are not feasible[4]. In the presence of metastases, sur
Most patients with advanced pNETs die due to tumor progression. Therefore, identifying new therapies with low toxicity and good tolerability to use concomitantly with the established pNET treatment is relevant. In this perspective, metformin is emerging as a molecule of interest[11-13].
Retrospective studies have described metformin, a widely used agent for the treatment of patients with type 2 diabetes mellitus (T2DM), to be effective in modulating different tumor-related events, including cancer incidence, recurrence and survival by inhibiting mTOR phosphorylation[14]. In pNET development, hyperactivation of PI3K/Akt signaling and activation of the mTOR pathway mediated through insulin-like growth factor-1 have been implicated to play a crucial role in carcinogenesis, thus providing the rationale for metformin use[15].
Moreover, although risk factors for pNET are still inconclusive, T2DM has been described as an essential contributor to tumor development, with a high incidence and prevalence of diabetes seen among pNET patients[16,17]. Indeed, the incidence of sporadic pNETs parallels that of T2DM, the highest in the fifth decade[18]. Moreover, T2DM, through chronic hyperglycemia, might accelerate tumor cell growth and spread, a mechanism seen in many cancer types[19], which might also negatively affect pNET prognosis[17], while metformin in vitro leads to inhibition of NET cell aggressiveness[20].
This systematic review evaluates the role of T2DM and metformin in the insurgence and post-treatment outcomes in patients with pNET. A pooled analysis was also performed according to the observed results.
This systematic review was conducted and reported under the guidelines for systematic reviews and meta-analyses for prognostic factor studies and the PRISMA and AMSTAR-2 Guidelines[21,22].
Objective: The main goal of this review was to systematically analyze and summarize evidence relating to the diagnostic and prognostic value of T2DM and metformin for predicting the insurgence and post-treatment outcomes of pNET.
Search strategy: Medline (PubMed) database was searched through June 2023 for relevant published original articles using the following keywords: (pancre AND neuroendocrine tumor) AND (diabetes OR T2DM OR mellit OR MODY OR DM2).
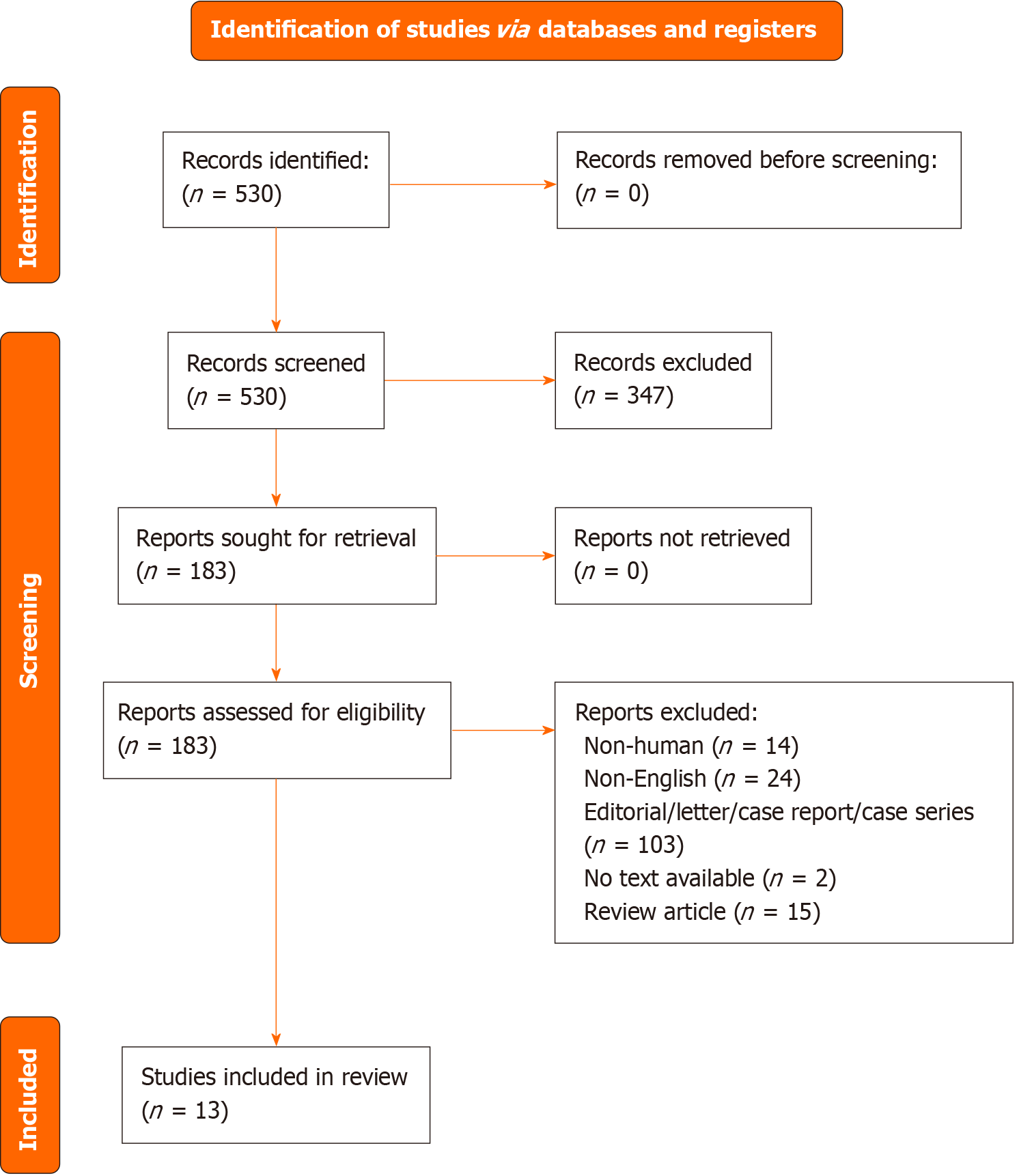
We also searched the reference lists of included studies. Two authors (Lai Q and Coppola A) independently reviewed the found records based on titles, abstracts, and the full text against the eligibility criteria (Figure 1). Consensus or a third reviewer (Cigrovski Berkovic M) resolved any conflict regarding study selection. Period of research: 01/01/2000-June 15, 2023.
Eligibility criteria: This review focused on retrospective and prospective observational studies that evaluated the diagnosis and the post-treatment outcomes in pNET adults over 18 years. Studies were included if they investigated the diagnostic or prognostic value of T2DM or metformin measured in pNET patients.
Case series, case reports, literature reviews or studies without adequate prognostic analyses were excluded. Studies were selected based on the PICOTS framework. No geographic or follow-up restrictions were applied. Only studies in the English language were considered. A limitation in the year of publication was applied, excluding all the studies before January 2000. If a study featured multiple eligible articles, we chose the most recent paper with the most significant number of participants and the most extended duration of follow-up.
Two independent reviewers (Lai Q and Coppola A) identified and collected data using the modified CHARMS-PF checklist[23]. Information extracted in each selected study included: First author (reference number), year of publication, country, period or study enrollment, design of the study, number of cases, number of controls, percentage of male sex, mean age, outcome measure, outcome value and 95%CI.
The Newcastle-Ottawa scale (NOS) was used to assess information on study quality; this scale varies from zero to a maximum possible score of nine and incorporates information on participant selection, outcome, exposure ascertainment, and the potential for confounding[24]. Two authors (Lai Q and Cigrovski Berkovic M) assessed the included studies. Any discrepancies were resolved by consensus or by a third reviewer (Sesa V).
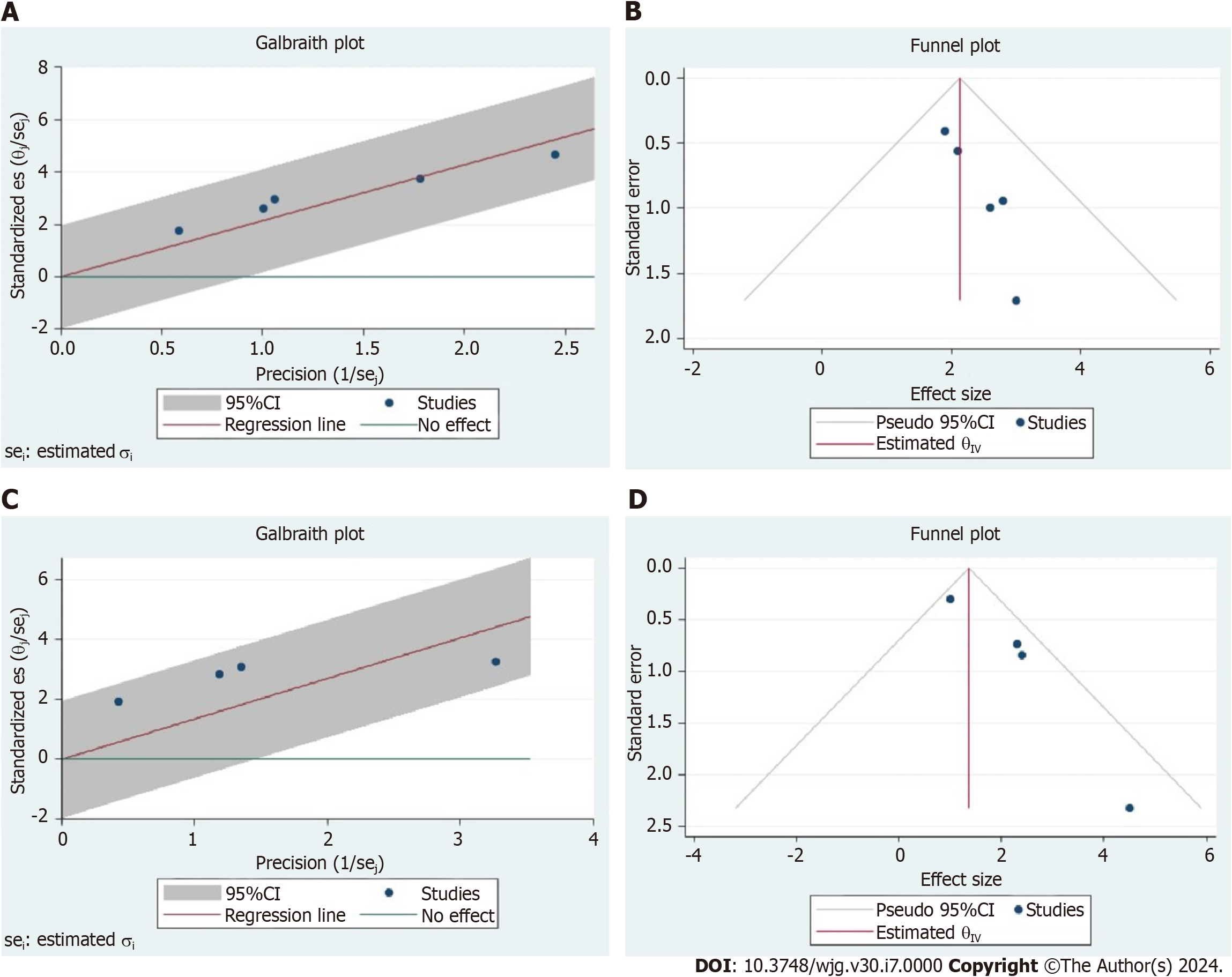
Odds ratios (ORs) or hazard ratios (HRs) with the corresponding 95%CI were used for the outcomes. Only the data adjusted for potential confounders were used to realize the pooled analyses reported in the present study. A random effects model was used to account for heterogeneity among studies. Heterogeneity was assessed using the Higgins I2 statistic[25]. An I2 > 75% indicated high heterogeneity, 50%–75% moderate heterogeneity, and < 50% mild heterogeneity[26]. Forest plots were used to graphically display the effect size in each study and the pooled estimates. The heterogeneity of the different studies was graphically reported using the Galbraith plot and the Funnel plot. A P value < 0.05 was considered statistically significant. All analyses were conducted using STATA statistical package version 14.0 (StataCorp LLC, College Station, TX, United States).
The PRISMA flow diagram summarizes the study selection process (Figure 1). The search strategy identified 530 records and no records from reference lists. Records were screened based on the selection by title/abstract. Three hundred forty-seven records were excluded because they were irrelevant to the review question or did not adhere to the inclusion criteria. Of the remaining 183 eligible records, 170 full-text articles were discarded for several reasons (Figure 1). In detail, the reasons for discard were: Non-human study (n = 14), non-English (n = 24), editorial/Letter/case report/case series (n = 103), no text available (n = 2), review article (n = 15), study not relevant (n = 12).
Key characteristics of the included studies are illustrated in Tables 1 and 2.
| Ref. | Country | Period | Design | n | Controls | Male (%) | Mean age (yr) | Outcome measure | 95%CI | NOS |
| Diagnosis | ||||||||||
| Hassan et al[27], 2008 | United States | 2000-2006 | Retro | 160 | 924 | 55 | 54 | OR | 2.8 (1.5–5.2) | 7 |
| Halfdanarson et al[28], 2014 | United States | 2000-2011 | Retro | 273 | 602 | 54 | 59 | OR | 1.9 (1.3-2.9) | 8 |
| Valente et al[29], 2017 | Europe | 2013-2015 | Retro | 201 | 603 | 51 | 60 | OR | 2.1 (1.3-3.5)1 | 8 |
| Giraldi et al[30], 2021 | Italy | 2014-2017 | Retro | 100 | 248 | 46 | NA | OR | 3.0 (1.2-7.9) | 8 |
| Feola et al[31], 2022 | Italy | NA | Retro | 75 | 210 | NA | NA | OR | 2.6 (1.3-5.2) | 6 |
| Treatment (PFS) | ||||||||||
| Pusceddu et al[32], 2018 | Italy | 1999-2015 | Retro | 445 | - | 53 | 59 | HR | 1.0 (0.6-1.5) | 8 |
| Pusceddu et al[33], 2021 | Italy | 2006-2013 | Pros | 204 | - | 52 | 62 | HR | 1.6 (1.0- 2.8) | 9 |
| Treatment (TFS) | ||||||||||
| de Mestier et al[34], 2020 | France | 2003-2018 | Retro | 268 | - | 40 | 55 | HR | 2.4 (1.2-4.5) | 8 |
| Sandini et al[35], 2020 | Germany | 2001-2017 | Retro | 417 | - | 56 | 58 | HR | 2.3 (1.3-4.2) | 8 |
| Fan et al[17], 2020 | China | 2006-2018 | Retro | 299 | - | 40 | NA | HR | 1.0 (0.6-1.8) | 8 |
| Tan et al[36], 2022 | China | 2009-2019 | Retro | 190 | - | 48 | NA | HR | 4.5 (1.2-10.3) | 8 |
| Treatment (OS) | ||||||||||
| Fan et al[17], 2020 | China | 2006-2018 | Retro | 299 | - | 40 | NA | HR | 1.2 (0.5-2.7) | 8 |
| Awwad et al[37], 2022 | Germany | 1999-2009 | Retro | 120 | - | NA | 58 | HR | 3.2 (1.2-10.3)2 | 8 |
| Zhang et al[38], 2022 | China | 2008-2020 | Retro | 335 | - | 43 | NA | HR | 2.7 (1.3-5.3) | 8 |
| Ref. | Country | Period | Design | n | Controls | Male (%) | Mean age (yr) | Outcome measure | 95%CI | NOS |
| Diagnosis | ||||||||||
| Hassan et al[27], 2008 | United States | 2000-2006 | Retro | 160 | 924 | 55 | 54 | OR | 0.8 (0.3-2.5) | 7 |
| Valente et al[29], 2017 | Europe | 2013-2015 | Retro | 201 | 603 | 51 | 60 | OR | 1.4 (0.7-2.7)1 1.5 (0.7-3.4)2 | 8 |
| Treatment (PFS) | ||||||||||
| Pusceddu et al[32], 2018 | Italy | 1999-2015 | Retro | 445 | - | 53 | 59 | HR | 0.5 (0.3-0.8) | 8 |
| Treatment (TFS) | ||||||||||
| Fan et al[17], 2020 | China | 2006-2018 | Retro | 299 | - | 40 | NA | HR | 0.8 (0.4-1.6)3 1.3 (0.6-3.0)3 | 8 |
| Treatment (OS) | ||||||||||
| Fan et al[17], 2020 | China | 2006-2018 | Retro | 299 | - | 40 | NA | HR | 0.7 (0.2-2.6)4 0.4 (0.1-2.8)4 | 8 |
| Awwad et al[37], 2022 | Germany | 1999-2009 | Retro | 120 | - | NA | 58 | HR | 2.6 (0.7-7.0)5 | 8 |
None of the studies included was a randomized controlled trial (RCT); only one was prospective, and the remaining 12 were retrospective experiences. No study reported was balanced after propensity score analysis.
Studies were conducted between 2008 and 2022 in five countries: Italy (n = 4), China (n = 3), the United States (n = 2), Germany (n = 2), and France (n = 1). One study was a European multicentric study.
The study population ranged from 120 to 1084 participants. The total number of cases enrolled was 5674 cases. The mean patient age range was 54-62 years, and the percentage of males ranged from 40%-56%. Heterogeneous outcomes were reported in the different studies. Five studies explored the role of T2DM as a risk factor for the insurgence of pNET[27-31], while the remaining eight studies explored the role of T2DM in terms of post-treatment outcomes[32-38]. The post-treatment outcomes were also heterogeneous, including progression-free survival (PFS), tumor-free survival (TFS), and overall survival (OS).
As for the role of metformin, only five studies explored its role in the setting of pNET[17,27,29,32,37]. In detail, two studies reported the role of metformin in the insurgence of pNET[27,29], and the remaining three explored PFS, TFS, or OS[17,32,37].
As reported in Tables 1 and 2, studies selected for review showed a good NOS, ranging from 6-9.
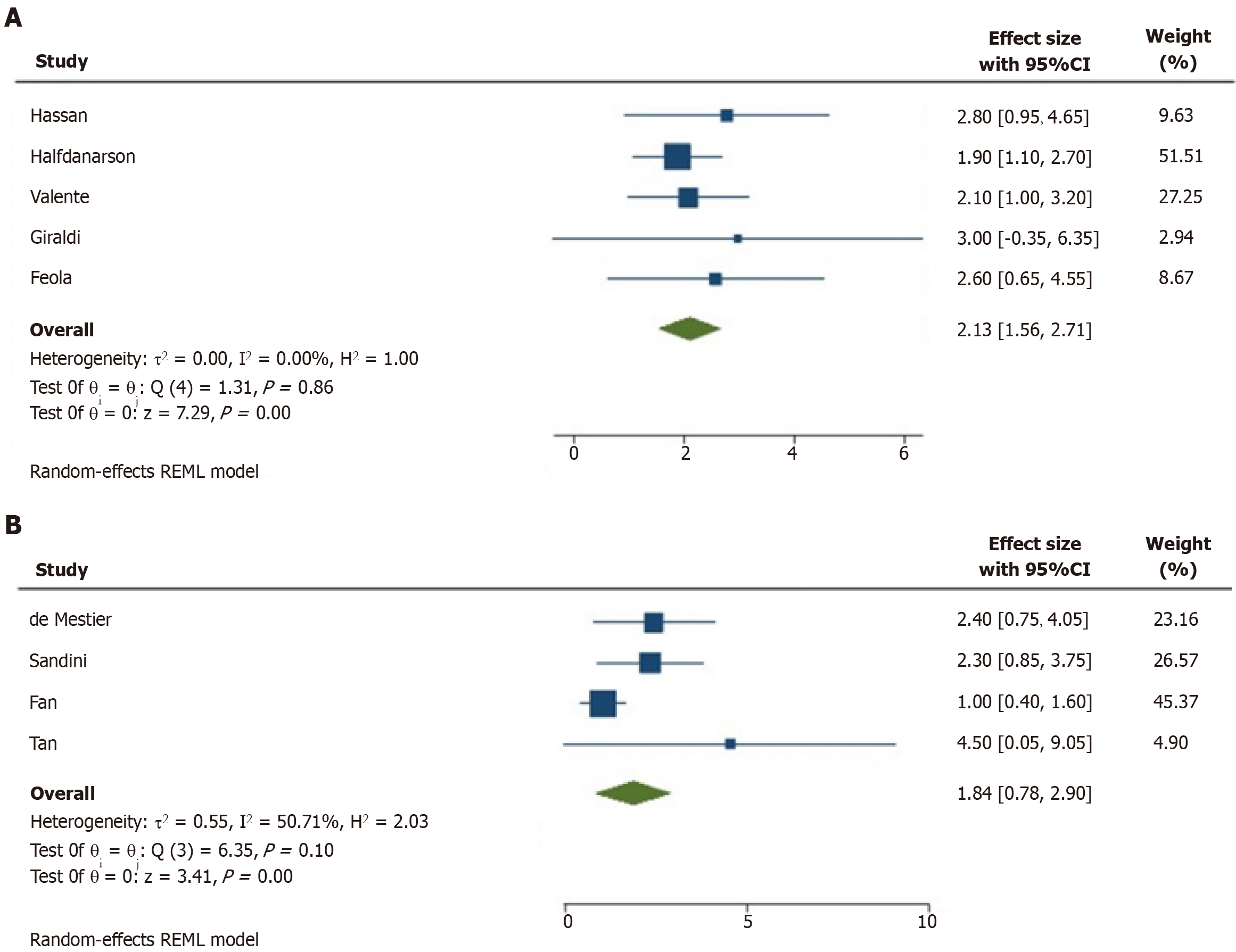
In all the studies exploring the role of T2DM as a risk factor for pNET insurgence, this disease always resulted as a risk factor[27-31]. A meta-analysis was performed to explore this aspect. In patients with T2DM, the risk for pNET insurgence was significantly increased (OR = 2.13, 95%CI = 1.56-4.55; P < 0.001). The heterogeneity of these studies was low, with an
Four studies explored the effect of T2DM in terms of post-treatment TFS[17,34-36]. The meta-analysis of HRs performed to explore this aspect showed that T2DM was a significant predictor of poor TFS (HR = 1.84, 95%CI = 0.78-2.90; P < 0.001). The heterogeneity of these studies was intermediate, with an I2 = 51% (Figure 2B). The intermediate heterogeneity was also graphically observable in the Galbraith and Funnel plots (Figure 3C and D).
The few studies exploring the different outcomes limited the possibility of performing pooled analyses in metformin. As reported in Table 2, heterogeneous results were reported, with no clear statistical relevance of the use of this drug in both the diagnosis and prognosis settings of pNET. Only one study showed a protective effect of metformin on the risk of PFS[32].
pNETs represent an increasingly diagnosed pancreatic pathology, with incidence rising with age[39].
According to population-based studies, 5-year survival of pNET patients, depending on patients' characteristics and the therapy used, ranges from 15% to 60%[40]. A minority of patients develop pNETs in association with inherited multiple endocrine neoplasia syndromes with known genetic mutations, while the majority of pNETs occur sporadically and knowledge of related risk factors is still insufficient. A study by Halfdanarson et al[28], investigating different risk factors among cases of low-grade pNETs, reported T2DM to be more common in pNET cases than matched controls (19% vs 11%, P < 0.001).
Our results performed on 3396 patients, including 809 pNET cases from five retrospective studies, confirm the correlation between T2DM and insurgence of pNET (OR = 2.13, 95%CI = 1.56-4.55; P < 0.001)[27-31]. Possible mechanisms are still speculative and involve both chronic hyperglycemia, which is a hallmark of T2DM and hyperinsulinemia. It seems that higher glucose availability to cancer cells, as present in T2DM, accelerates tumor growth, proliferation and metastatic spread, while hyperinsulinemia might further promote tumor growth through direct and indirect effects. As a direct effect, insulin stimulates glucose uptake and consumption by the pNET cells, stimulating their proliferation and indirectly, insulin displays mitogenic actions promoting cell division and spread and inhibiting apoptosis through the activation of the IR-IGF-1-receptor/PI3K/AKT/mTORC1 pathway. Moreover, hyperinsulinemia downregulates the expression of IGF-1-BPs, which, in turn, enhances the bioavailability of IGF-1 and promotes its binding to IGF1R, leading to tumor cell growth[41]. In addition, low-grade chronic inflammation accompanying T2DM can also create a beneficial tumor microenvironment, promoting pNET growth and spread[42,43]. A few studies (mainly retrospective) have also reported the correlation between T2DM and the prognosis of pNETs. According to a study by Fan and coworkers, in the case of concomitant T2DM and pNET, patients had a greater chance for metastatic disease and neural invasion[17], greater tumor size[44] and poor survival post-pancreatic surgery[35]. We analyzed the pooled data from 1174 pNET patients and found the correlation between T2DM and TFS in pNET patients (HR = 1.84, 95%CI = 0.78-2.90; P < 0.001), suggesting higher recurrence risk in case of concomitant T2DM[17,32-38].
As T2DM seems to be a risk factor for contracting pNET and potentially negatively impacts the patients' outcomes, studies exploring the role of anti-diabetic agents, specifically metformin, in similar settings are of importance. Metformin has been investigated as an anticancer agent in the setting of different cancer types. In the case of pancreatic adenocarcinoma, its use in diabetic patients was associated with reduced cancer risk, while data on patients' survival are still inconclusive but also suggestive of positive effects[45]. The possibility to repurpose metformin in case of pNET treatment is suggested by the results of a small study by Pusceddu et al[46] where 12 patients with advanced G 1–2 pNETs and concomitant T2DM (compared to 19 patients without T2DM) had a significantly longer PFS if treated with metformin on top of everolimus 10 mg daily in combination with octreotide LAR 30 mg i.m. every 28 d. Median PFS was 29 mo in patients with T2DM taking metformin compared with 11 mo in normoglycemic patients (P = 0.018)[46]. A more extensive multicentric Italian study involving 445 patients with advanced pNETs suggests metformin, probably irrespective of its dose, significantly prolongs PFS of patients with T2DM compared to other anti-diabetic drugs used on top of everolimus with or without somatostatin analogs (44.2 mo vs 20.8 mo), especially if introduced three months prior to standard anticancer treatment[32].
The post hoc analysis of the CLARINET study, including patients with advanced, non-functional entero-pancreatic NETs with an indolent course (both pNETs and intestinal NETS with a Ki67 ≤ 10%) treated with lanreotide or placebo also showed a favorable effect of metformin on the PFS of patients who had T2DM prior to study treatment and were randomized to the placebo arm. In this patient subgroup, PFS more than doubled compared to patients not receiving metformin. On the other hand, there was no additional benefit when metformin was added to patients treated with lanreotide[33].
Currently, evidence from prospective, randomized studies is still not available. Until the data from the ongoing clinical trial, MetNET1[47], a prospective, open-label, single-arm trial in which patients with advanced pNETs will receive metformin in combination with first-line somatostatin analogs and everolimus, also including patients without diabetes mellitus based on published preclinical data indicating that metformin also produces direct (cell-autonomous) antitumor effects, independent of glucose extracellular concentration becomes public there is no firm recommendation for its use in the setting of pNET[10,20].
In this analysis, we could only include a small number of studies, limiting the possibility of performing pooled analysis. The heterogeneity of included studies enabled us to explore its relevance in the settings of diagnosis and prognosis of pNETs.
The present study presents some limitations. Only one prospective study was available, and no RCTs were present among the investigable studies. Therefore, heterogeneity across the studies and potential inclusion biases should be considered. Second, it was impossible to perform detailed pooled analyses concerning several outcomes due to the paucity of studies to consider. This limitation was particularly true in the case of metformin studies. Lastly, several potential confounders that are impossible to analyze should be considered, like the duration of T2DM, the concomitant use of insulin or the duration of anti-diabetic therapies. This type of data should be relevant in constructing meta-regressions, but unfortunately, these data were missing in several explored studies.
In conclusion, until results of RCTs, including patients with pNETs with or without concomitant T2DM receiving metformin in different proven anticancer treatments, become available, data on metformin effects in this setting is still inconclusive.
T2DM represents a risk factor for the insurgence of pNET and is a significant predictor of poor post-treatment TFS of pNET patients. Unfortunately, a few studies with heterogeneous results limited the possibility of exploring the effect of metformin in the diagnosis and prognosis of pNET.
Advanced pancreatic neuroendocrine tumors (pNETs) are difficult to treat with low overall survival (OS). In pNET development, hyperactivation of PI3K/Akt signaling and activation of the mTOR pathway mediated through insulin-like growth factor-1 have been implicated to play a crucial role in carcinogenesis, thus providing the rationale for metformin use. Moreover, although risk factors for pNET are still inconclusive, type 2 Diabetes mellitus (T2DM) has been described as an essential contributor to tumor development, with a high incidence and prevalence of diabetes seen among pNET patients. This systematic review evaluates the role of T2DM and metformin in the insurgence and post-treatment outcomes in patients with pNET.
Regarding scarce data on pNET treatment and risk factors we wanted to investigate and analyze available data related to diagnostic and prognostic value of T2DM and metformin in association with pNET.
The main aim of this review was to systematically analyze and summarize evidence related to the diagnostic and prognostic value of T2DM and metformin for predicting the insurgence and post-treatment outcomes of pNETs.
A systematic review of the published literature was undertaken, focusing on the role of T2DM and metformin in insurgence and prognosis of pNET, measured through outcomes of tumor-free survival (TFS), OS and progression-free survival.
A total of 13 studies (n = 5674 patients) were included in this review. Analysis of 809 pNET cases from five retrospective studies (low study heterogeneity with I2 = 0%) confirms the correlation between T2DM and insurgence of pNET (odds ratios = 2.13, 95%CI = 1.56-4.55; P < 0.001). The pooled data from 1174 pNET patients showed the correlation between T2DM and post-treatment (TFS) in pNET patients (hazard ratio = 1.84, 95%CI = 0.78-2.90; P < 0.001). The study heterogeneity was intermediate, with I2 = 51%. A few studies limited the possibility of performing pooled analysis in the setting of metformin; therefore, results were heterogeneous, with no statistical relevance to the use of this drug in the diagnosis and prognosis of pNET.
T2DM represents a risk factor for the insurgence of pNET and is a significant predictor of poor post-treatment (TFS) of pNET patients. Unfortunately, a few studies with heterogeneous results limited the possibility of exploring the effect of metformin in the diagnosis and prognosis of pNET.
Future research should further try to identify other risk factors and their influence on pNETs as well as the role of metformin in the diagnosis and prognosis of pNET.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu D, China S-Editor: Lin C L-Editor: A P-Editor: Zhao YQ
| 1. | Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (1)] |
| 2. | Orditura M, Petrillo A, Ventriglia J, Diana A, Laterza MM, Fabozzi A, Savastano B, Franzese E, Conzo G, Santini L, Ciardiello F, De Vita F. Pancreatic neuroendocrine tumors: Nosography, management and treatment. Int J Surg. 2016;28 Suppl 1:S156-S162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 405] [Article Influence: 57.9] [Reference Citation Analysis (1)] |
| 4. | Sonbol MB, Mazza GL, Mi L, Oliver T, Starr J, Gudmundsdottir H, Cleary SP, Hobday T, Halfdanarson TR. Survival and Incidence Patterns of Pancreatic Neuroendocrine Tumors Over the Last 2 Decades: A SEER Database Analysis. Oncologist. 2022;27:573-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1290] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 6. | Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller HH, Arnold R; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 7. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2117] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 8. | Vinik A, Bottomley A, Korytowsky B, Bang YJ, Raoul JL, Valle JW, Metrakos P, Hörsch D, Mundayat R, Reisman A, Wang Z, Chao RC, Raymond E. Patient-Reported Outcomes and Quality of Life with Sunitinib Versus Placebo for Pancreatic Neuroendocrine Tumors: Results From an International Phase III Trial. Target Oncol. 2016;11:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Starr JS, Sonbol MB, Hobday TJ, Sharma A, Kendi AT, Halfdanarson TR. Peptide Receptor Radionuclide Therapy for the Treatment of Pancreatic Neuroendocrine Tumors: Recent Insights. Onco Targets Ther. 2020;13:3545-3555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Vitali E, Boemi I, Tarantola G, Piccini S, Zerbi A, Veronesi G, Baldelli R, Mazziotti G, Smiroldo V, Lavezzi E, Spada A, Mantovani G, Lania AG. Metformin and Everolimus: A Promising Combination for Neuroendocrine Tumors Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, Crowe FL, Farmer AJ, Harrison S, Hirst JA, Home P, Kahn SE, McLellan JH, Perera R, Plüddemann A, Ramachandran A, Roberts NW, Rose PW, Schweizer A, Viberti G, Holman RR. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 807] [Cited by in RCA: 1015] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 13. | Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60:1639-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 14. | Schulten HJ. Pleiotropic Effects of Metformin on Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Vitali E, Boemi I, Piccini S, Tarantola G, Smiroldo V, Lavezzi E, Brambilla T, Zerbi A, Carnaghi C, Mantovani G, Spada A, Lania AG. A novel insight into the anticancer mechanism of metformin in pancreatic neuroendocrine tumor cells. Mol Cell Endocrinol. 2020;509:110803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Haugvik SP, Hedenström P, Korsæth E, Valente R, Hayes A, Siuka D, Maisonneuve P, Gladhaug IP, Lindkvist B, Capurso G. Diabetes, smoking, alcohol use, and family history of cancer as risk factors for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Neuroendocrinology. 2015;101:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Fan Z, Gong Y, Huang Q, Yang C, Cheng H, Jin K, Fan K, Ni Q, Yu X, Luo G, Liu C. Diabetes Is Associated With the Metastasis of Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Öberg K, Knigge U, Kwekkeboom D, Perren A; ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii124-vii130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 19. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11811] [Article Influence: 738.2] [Reference Citation Analysis (0)] |
| 20. | Herrera-Martínez AD, Pedraza-Arevalo S, L-López F, Gahete MD, Gálvez-Moreno MA, Castaño JP, Luque RM. Type 2 Diabetes in Neuroendocrine Tumors: Are Biguanides and Statins Part of the Solution? J Clin Endocrinol Metab. 2019;104:57-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4127] [Cited by in RCA: 4682] [Article Influence: 1170.5] [Reference Citation Analysis (0)] |
| 22. | Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, Hayden J, Collins GS, Debray TPA. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 23. | Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1144] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 24. | The Ottawa Hospital. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [cited 10 October 2023]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 25. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25802] [Article Influence: 1121.8] [Reference Citation Analysis (0)] |
| 26. | Lai Q, Giovanardi F, Mennini G, Berardi G, Rossi M. The impact of mini-invasive right hepatectomy in the setting of living donation: a meta-analysis. Updates Surg. 2022;74:23-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Hassan MM, Phan A, Li D, Dagohoy CG, Leary C, Yao JC. Risk factors associated with neuroendocrine tumors: A U.S.-based case-control study. Int J Cancer. 2008;123:867-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Halfdanarson TR, Bamlet WR, McWilliams RR, Hobday TJ, Burch PA, Rabe KG, Petersen GM. Risk factors for pancreatic neuroendocrine tumors: a clinic-based case-control study. Pancreas. 2014;43:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Valente R, Hayes AJ, Haugvik SP, Hedenström P, Siuka D, Korsæth E, Kämmerer D, Robinson SM, Maisonneuve P, Delle Fave G, Lindkvist B, Capurso G. Risk and protective factors for the occurrence of sporadic pancreatic endocrine neoplasms. Endocr Relat Cancer. 2017;24:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Giraldi L, Vecchioni A, Carioli G, Bilotta M, La Rosa S, Imperatori A, Volante M, Brizzi MP, Inzani F, Petrone G, Schinzari G, Bianchi A, Margaritora S, Alfieri S, La Vecchia C, Boccia S, Rindi G. Risk factors for pancreas and lung neuroendocrine neoplasms: a case-control study. Endocrine. 2021;71:233-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Feola T, Puliani G, Sesti F, Modica R, Centello R, Minotta R, Cannavale G, Di Meglio S, Di Vito V, Lauretta R, Appetecchia M, Colao A, Lenzi A, Isidori AM, Faggiano A, Giannetta E. Risk factors for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): a three-centric case-control study. J Endocrinol Invest. 2022;45:849-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Pusceddu S, Vernieri C, Di Maio M, Marconcini R, Spada F, Massironi S, Ibrahim T, Brizzi MP, Campana D, Faggiano A, Giuffrida D, Rinzivillo M, Cingarlini S, Aroldi F, Antonuzzo L, Berardi R, Catena L, De Divitiis C, Ermacora P, Perfetti V, Fontana A, Razzore P, Carnaghi C, Davì MV, Cauchi C, Duro M, Ricci S, Fazio N, Cavalcoli F, Bongiovanni A, La Salvia A, Brighi N, Colao A, Puliafito I, Panzuto F, Ortolani S, Zaniboni A, Di Costanzo F, Torniai M, Bajetta E, Tafuto S, Garattini SK, Femia D, Prinzi N, Concas L, Lo Russo G, Milione M, Giacomelli L, Buzzoni R, Delle Fave G, Mazzaferro V, de Braud F. Metformin Use Is Associated With Longer Progression-Free Survival of Patients With Diabetes and Pancreatic Neuroendocrine Tumors Receiving Everolimus and/or Somatostatin Analogues. Gastroenterology. 2018;155:479-489.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Pusceddu S, Vernieri C, Di Maio M, Prinzi N, Torchio M, Corti F, Coppa J, Buzzoni R, Di Bartolomeo M, Milione M, Regnault B, Truong Thanh XM, Mazzaferro V, de Braud F. Impact of Diabetes and Metformin Use on Enteropancreatic Neuroendocrine Tumors: Post Hoc Analysis of the CLARINET Study. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | de Mestier L, Védie AL, Faron M, Cros J, Rebours V, Hentic O, Do Cao C, Bardet P, Lévy P, Sauvanet A, Ruszniewski P, Hammel P. The Postoperative Occurrence or Worsening of Diabetes Mellitus May Increase the Risk of Recurrence in Resected Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2020;110:967-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Sandini M, Strobel O, Hank T, Lewosinska M, Nießen A, Hackert T, Büchler MW, Schimmack S. Pre-operative dysglycemia is associated with decreased survival in patients with pancreatic neuroendocrine neoplasms. Surgery. 2020;167:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Tan Q, Wang X, Chen C, Liu X, Chen Y, Tan C. Prognostic value of preoperative diabetes mellitus in patients with non-functional pancreatic neuroendocrine neoplasms. Am J Surg. 2022;224:1162-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Awwad F, Ozga AK, Amin T, Schlueter C, Kailani S, Perez D, Wolter S, Sauter G, Izbicki J, Lohse AW, Schrader J. Metabolic Syndrome Is Associated with Impaired Survival after Surgery for Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2022;112:1225-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Zhang P, Xiao Z, Xu H, Zhu X, Wang L, Huang D, Liang Y, Ni Q, Chen J, Yu X, Luo G. Hyperglycemia is associated with adverse prognosis in patients with pancreatic neuroendocrine neoplasms. Endocrine. 2022;77:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 623] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 40. | Strosberg JR, Cheema A, Weber J, Han G, Coppola D, Kvols LK. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29:3044-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 41. | Kumar S, Senapati S, Bhattacharya N, Bhattacharya A, Maurya SK, Husain H, Bhatti JS, Pandey AK. Mechanism and recent updates on insulin-related disorders. World J Clin Cases. 2023;11:5840-5856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 42. | Ben Q, Zhong J, Fei J, Chen H, Yv L, Tan J, Yuan Y. Risk Factors for Sporadic Pancreatic Neuroendocrine Tumors: A Case-Control Study. Sci Rep. 2016;6:36073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Herman Mahečić D, Cigrovski Berković M, Zjačić-Rotkvić V, Čačev T, Kapitanović S, Ulamec M. Inflammation-related cytokines and their roles in gastroenteropancreatic neuroendocrine neoplasms. Bosn J Basic Med Sci. 2020;20:445-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Zhuge X, Wang Y, Chen X, Guo C. Diabetes in Patients With Pancreatic Neuroendocrine Neoplasms. Front Endocrinol (Lausanne). 2020;11:615082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Xin W, Fang L, Fang Q, Zheng X, Huang P. Effects of metformin on survival outcomes of pancreatic cancer patients with diabetes: A meta-analysis. Mol Clin Oncol. 2018;8:483-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Pusceddu S, Buzzoni R, Vernieri C, Concas L, Marceglia S, Giacomelli L, Milione M, Leuzzi L, Femia D, Formisano B, Mazzaferro V, de Braud F. Metformin with everolimus and octreotide in pancreatic neuroendocrine tumor patients with diabetes. Future Oncol. 2016;12:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Pusceddu S, de Braud F, Concas L, Bregant C, Leuzzi L, Formisano B, Buzzoni R. Rationale and protocol of the MetNET-1 trial, a prospective, single center, phase II study to evaluate the activity and safety of everolimus in combination with octreotide LAR and metformin in patients with advanced pancreatic neuroendocrine tumors. Tumori. 2014;100:e286-e289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |