Published online Feb 21, 2024. doi: 10.3748/wjg.v30.i7.728
Peer-review started: October 21, 2023
First decision: December 5, 2023
Revised: December 18, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: February 21, 2024
Processing time: 123 Days and 3.1 Hours
Liver injury is common in severe acute pancreatitis (SAP). Excessive autophagy often leads to an imbalance of homeostasis in hepatocytes, which induces lipid peroxidation and mitochondrial iron deposition and ultimately leads to ferrop
To speculate whether MFG-E8 could also alleviate SAP induced liver injury by restoring the abnormal autophagy flux.
SAP was induced in mice by 2 hly intraperitoneal injections of 4.0 g/kg L-arginine or 7 hly injections of 50 μg/kg cerulein plus lipopolysaccharide. mfge8-knockout mice were used to study the effect of MFG-E8 deficiency on SAP-induced liver injury. Cilengitide, a specific αvβ3/5 integrin inhibitor, was used to investigate the possible mechanism of MFG-E8.
The results showed that MFG-E8 deficiency aggravated SAP-induced liver injury in mice, enhanced autophagy flux in hepatocyte, and worsened the degree of ferroptosis. Exogenous MFG-E8 reduced SAP-induced liver injury in a dose-dependent manner. Mechanistically, MFG-E8 mitigated excessive autophagy and inhibited ferroptosis in liver cells. Cilengitide abolished MFG-E8’s beneficial effects in SAP-induced liver injury.
MFG-E8 acts as an endogenous protective mediator in SAP-induced liver injury. MFG-E8 alleviates the excessive autophagy and inhibits ferroptosis in hepatocytes by binding to integrin αVβ3/5.
Core Tip: Serum milk fat globule epidermal growth factor 8 (MFG-E8) concentration is negatively correlated with inflammatory severity in acute pancreatitis (AP) patients. Deficiency of MFG-E8 would aggravate the hepatic inflammatory response, and exacerbates the imbalance of liver autophagy flux caused by AP. Eventually leading to ferroptosis. Recombinant MFG-E8 administration restored autophagy flux, reduced ferroptosis and alleviated liver injury in AP in a dose-dependent manner. MFG-E8 alleviates the excessive autophagy and inhibits ferroptosis in hepatocytes possibly by binding to integrin αVβ3/5.
- Citation: Cui Q, Liu HC, Liu WM, Ma F, Lv Y, Ma JC, Wu RQ, Ren YF. Milk fat globule epidermal growth factor 8 alleviates liver injury in severe acute pancreatitis by restoring autophagy flux and inhibiting ferroptosis in hepatocytes. World J Gastroenterol 2024; 30(7): 728-741
- URL: https://www.wjgnet.com/1007-9327/full/v30/i7/728.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i7.728
Severe acute pancreatitis (SAP) is a systemic disease characterized by pancreatic self-digestion and a systemic inflammatory response[1,2]. Compared to the self-limiting nature of mild AP, multiple organ dysfunction syndrome (MODS) caused by SAP develops cascade exacerbations without intervention, and it is one of the leading causes of death in the emergency department of digestive diseases[3,4]. As an organ adjacent to the pancreas, the liver is often the first echelon of external pancreatic attack caused by SAP. The metabolic and exocrine dysfunction caused by liver injury further aggravates the failure of other vital organs, forming a second hit for the patient. The invasion of peripancreatic necrosis products combined with hematogenous spread of intestinal bacteria results in destruction of the liver parenchyma[5,6]. Disorders of liver cell metabolism and exocrine function further aggravate the failure of the lung, kidney and other important organs, forming the second hit after the onset of SAP[7,8].
Autophagy is a catabolic process in which autophagic lysosomes degrade most cytoplasmic contents[9]. Stable autophagic flux participates in maintaining the normal homeostasis of cells, such as hepatocytes. Autophagy involves proteins encoded by a group of autophagy-related genes (ATGs)[10]. Mammalian autophagy related protein 16 like protein 1 (ATG16L1) contains a coiled-coil domain with an amino terminal and multiple carboxy-terminal WD repeats. ATG16L1 has the ability to lipidate LC3-binding sites and maintain autophagy circulation[11,12]. Abnormal protein expression of ATGs combined with increased LC3I to LC3II transformation and depletion of autophagy substrate transporters (e.g., P62) suggests hyperactivation of intracellular autophagy.
Excessive autophagy leads to ferroptosis, which is a new type of iron-dependent programmed cell death that is different from apoptosis and necrosis[13-15]. The main mechanism of ferroptosis is that under the action of divalent iron or ester oxygenase, unsaturated fatty acids with high expression on the cell membrane are catalyzed to produce lipid peroxidation, thus inducing cell death. In addition, a decrease in glutathione peroxidase 4 (GPX4), the core enzyme regulating the antioxidant system (glutathione system), is observed[16-18]. Disturbances in intracellular homeostasis, including hyperactivity of autophagy, thereby lead to ferroptosis. Active ferroptosis does not promote the recovery of cell function but aggravates the damage. Recent evidence suggests that ferroptosis contributes to acute and chronic liver injury and that it may be one of the major culprits of liver cell death[19-21]. Therefore, we focused on the changes in hepatocyte autophagy and ferroptosis in liver injury caused by SAP and explored possible therapeutic targets.
Milk fat globule epidermal growth factor 8 (MFG-E8) is a secreted lipophilic glycoprotein that contains an RGD motif and interacts with integrins[22,23]. MFG-E8 is involved in a variety of intercellular signaling pathways and enhances the phagocytosis of inflammatory cells and apoptotic cells by macrophages[24-26]. Our previous study confirmed that MFG-E8 maintains cellular homeostasis by suppressing endoplasmic reticulum stress (ERS) in pancreatic exocrine acinar cells and protects against SAP[27,28]. ERS has been reported to play an important role in promoting intracellular autophagy disorder and inducing ferroptosis[29]. MFG-E8 also plays a protective role in nonalcoholic fatty liver disease[30]. However, whether MFG-E8 also plays a role in liver injury caused by SAP remains unknown. Therefore, the present study aimed to clarify the specific role of MFG-E8 in SAP-induced liver injury and its regulation of homeostasis in damaged liver cells.
In total, 134 AP patients (age ≥ 18 years) who were treated at the First Affiliated Hospital of Xi’an Jiaotong University from January 2018 to January 2019 were included in this study. AP was diagnosed according to the International Atlanta Symposium on Acute Pancreatitis[1]. The present study was approved by the Ethics Committee of First Affiliated Hospital of Xi’an Jiaotong University.
MFG-E8 knockout mice purchased from Shanghai Model Organisms Center (Shanghai, China) were used in this study. The litter wild-type mice were used as the control group in this experiment. The male adult C57BL/6J mice used in the exogenous MFG-E8 experiment were purchased from the Animal Experimental Center of Xi’an Jiaotong University (Xi’an, China). All laboratory animals were housed in a standard animal laboratory facility that simulates day and night on 12-h cycles. The mice had free access to water and food, and each cage was equipped with its own ventilation system. Before experimental intervention, all mice were randomly divided into groups with six mice per group and fasted for one night. The study protocol was approved by the Institutional Animal Care and Use Committee of the Ethics Committee of Xi’an Jiaotong University Health Science Center.
L-arginine-SAP was induced by 2 hly intraperitoneal injections of 4.0 g/kg L-arginine (Sigma Aldrich, Shanghai, China). At 2 h after the last injection of L-arginine, normal saline (vehicle) or 5, 10, or 20 μg/kg MFG-E8 (RD System, Inc. Minnesota, United States) was administered through intraperitoneal injection. To determine the role of the αvβ3/5 integrins in the effect of MFG-E8 on the liver, cilengitide (20 mg/kg, SELLECK, Texas, United States), a specific αvβ3/5 integrin inhibitor[31,32], was administered through intraperitoneal injection at 1 h after the last injection of L-arginine. The animals were anesthetized by inhalation of isoflurane and sacrificed at 69 h after MFG-E8 treatment (i.e., 72 h after the first injection of L-arginine). Serum and hepatic tissue samples were collected.
Cerulein + lipopolysaccharide (LPS)-SAP was induced by 7 hly injections of cerulein (50 μg/kg). LPS (10 mg/kg, L8880, Solarbio, Beijing, China) was added to the last cerulein injection. At 30 min after the second injection of cerulein, 20 μg/kg MFG-E8 was administered intraperitoneally. Blood and tissue samples were harvested at 4 h after the last injection of cerulein (i.e., 11 h after the first injection of cerulein).
Detailed methods for the following procedures and antibody information are provided in the Supplementary materials: Hematoxylin and eosin (H&E) staining; enzyme-linked immunosorbent assay; granulocyte myeloperoxidase (MPO) assessment; detection of glutathione (GSH) and malondialdehyde (MDA) levels; TdT-mediated dUTP Nick-End Labeling (TUNEL) staining; transmission electron microscopy; western blot analysis; antibodies and statistical analysis.
Systemic inflammation caused by AP often leads to MODS and is the culprit of liver damage. Our previous study found that the concentration of MFG-E8 in the serum of AP patients is inversely proportional to the severity of the disease[27]. In the present study, we further explored the potential correlation between serum MFG-E8 concentration and the severity of systemic inflammation. A total of 134 AP patients were included in this study. Of these patients, 57 patients (42.5%) had biliary disease, 5 patients (3.7%) had alcohol misuse, 37 patients (27.6%) had hyperlipidemia and 35 patients (26.1%) had other causes. Local complications, organ failure and in-hospital mortality occurred in 35 patients (26.1%), 19 patients (14.2%), and 1 patient (0.7%), respectively (Supplementary Table 1).
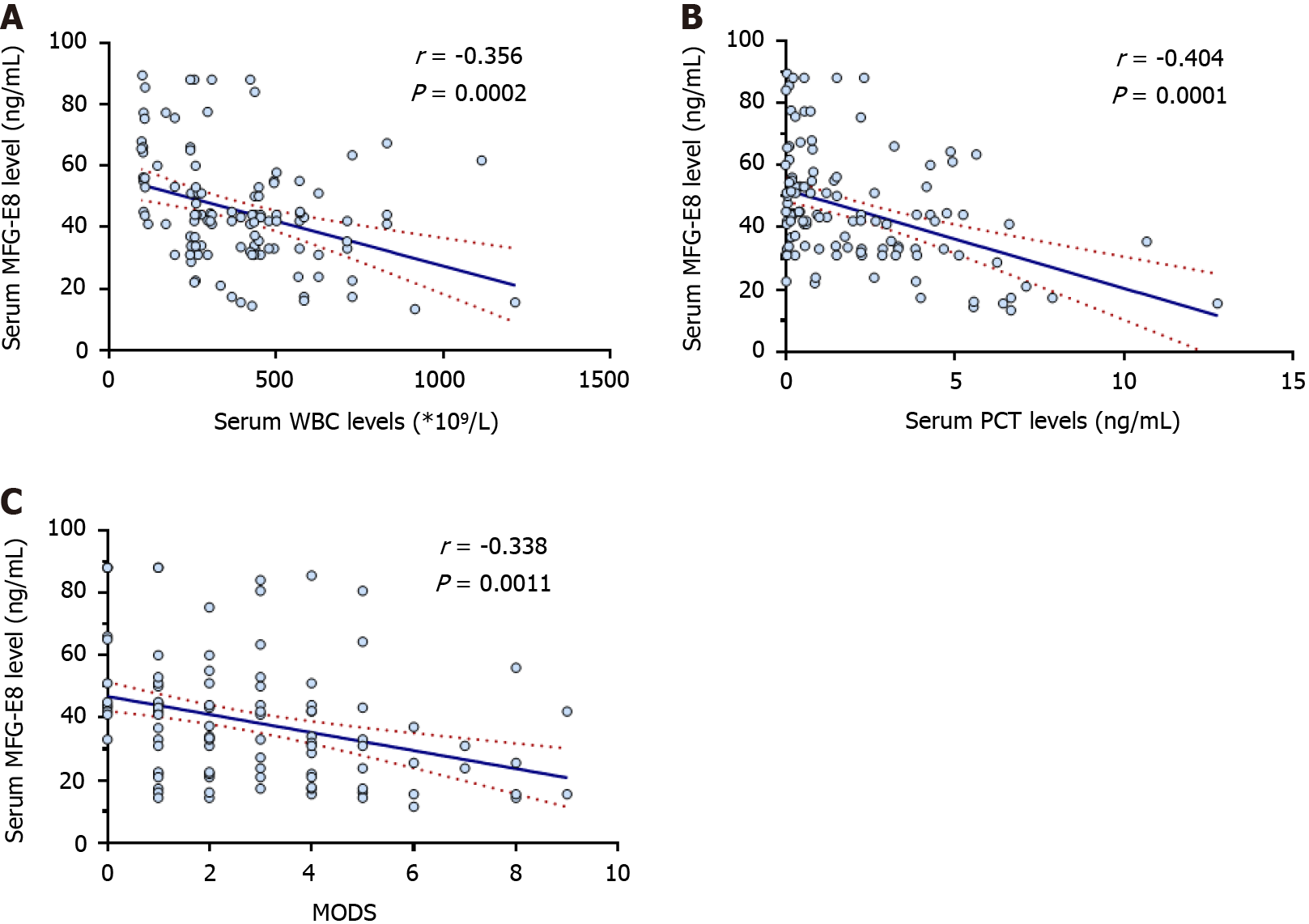
White blood cells (WBCs) and procalcitonin (PCT) are commonly used circulating markers of inflammation. As shown in Figure 1A and B, serum MFG-E8 concentrations were negatively correlated with serum WBC counts (r = -0.356, P < 0.01) and PCT concentrations (r = -0.404, P < 0.01), suggesting an inverse relationship between serum MFG-E8 levels and inflammatory severity. Furthermore, serum MFG-E8 concentrations were negatively correlated with MODS scores[33] (r = -0.338, P < 0.01) in AP patients (Figure 1C). These results suggested that AP patients with low concentrations of MFG-E8 are more prone to sepsis and MODS.
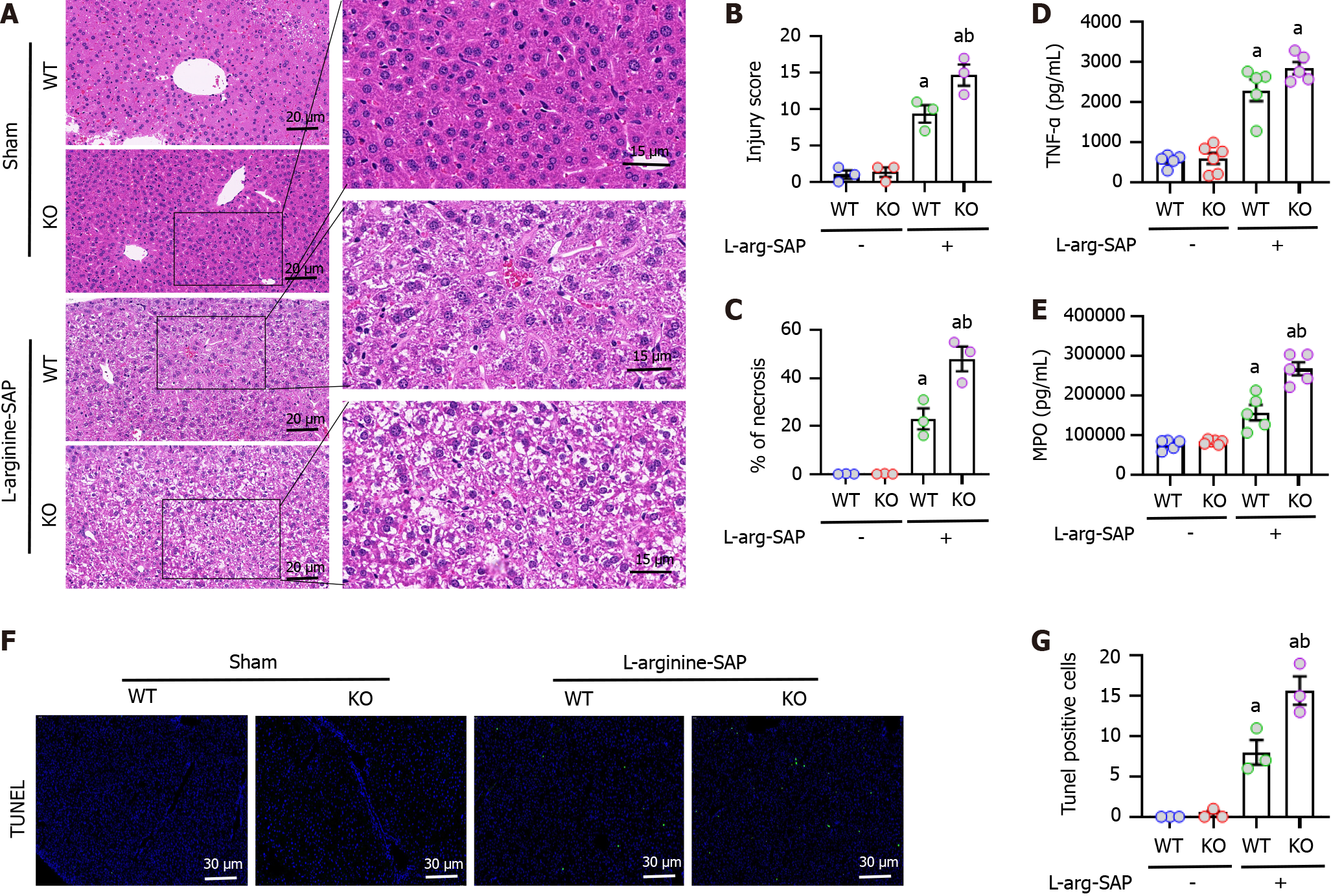
SAP was induced in mice by intraperitoneal injection of L-arginine. Compared to control mice, the liver of SAP mice was significantly damaged with structural disorder of liver lobules, vacuoles in liver cells and accumulation of red blood cells in hepatic sinusoids (Figure 2A and B). However, knockout of mfge8 gene resulted in more significant liver damage in SAP mice, and the degree of liver damage in MFG-E8-deficient mice was 0.3 times worse than that in wild-type mice after quantification of the pathological score (P < 0.05, Figure 2A and B). The area of liver necrosis in MFG-E8-deficient mice was twice as much as that in wild-type mice (P < 0.05, Figure 2C).
Our previous studies confirmed that SAP leads to impaired intestinal mucosal barrier function, resulting in intestinal bacteria transfer into the blood and inducing multiple organ infections[34]. To examine whether the liver is also secondary to inflammation in experimental SAP, we examined the expression levels of a range of cytokines in the liver. As shown in Figure 2D and E, the expression levels of tumor necrosis factor (TNF)-α and MPO were significantly higher than normal in the liver of SAP mice, which was exacerbated by the loss of MFG-E8 expression. These results suggested that MFG-E8 deficiency may reduce the anti-inflammatory ability of the liver in experimental SAP (P < 0.05). Consistent with the pathological scores, there was a greater proportion of apoptotic cells labeled with TUNEL fluorescence in MFG-E8-deficient SAP mice, which was more than 1.5 times that of wild-type SAP mice (P < 0.05, Figure 2F and G).
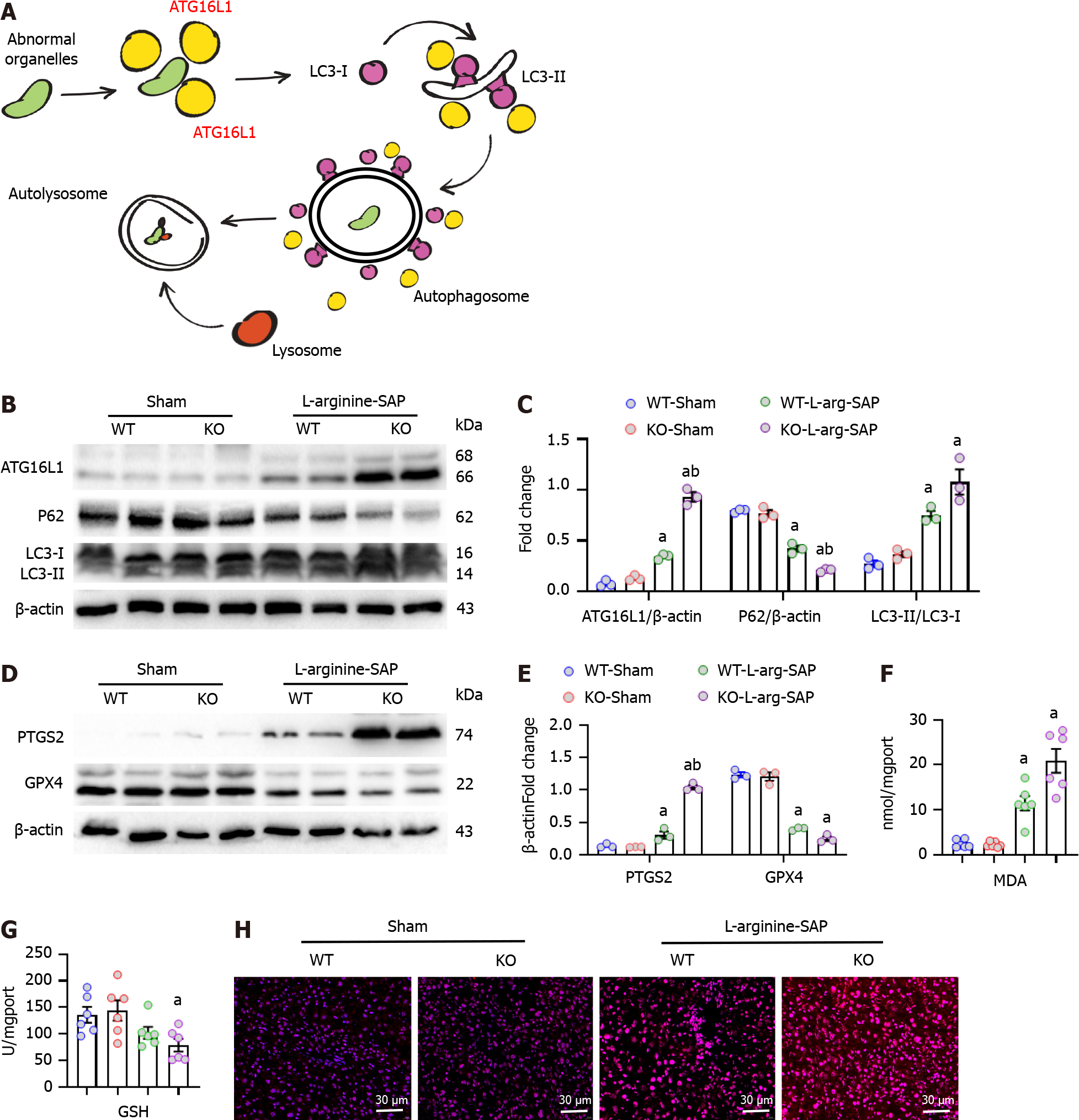
Excessive autophagy is secondary to the damage of hepatocytes caused by external physical and chemical factors, and the hyperactive autophagic flux further promotes the imbalance of cellular homeostasis[35,36]. Therefore, we detected the expression levels of the autophagy-related proteins, ATG16L1, P62 and LC3B in the liver. ATG16L1 played a crucial role in determining the lipidylation site of LC3 and promoting the maturation of autophagosomes[11] (Figure 3A), while the lack of MFG-E8 increased the level of ATG16L1 in the liver of SAP mice (P < 0.05, Figure 3B and C). P62 and LC3 cooperatively constitute the ability of autophagosomes to form, degrade and transport degradation substrates[37]. As shown in Figure 3B and C, SAP resulted in abnormal expression levels of P62 and LC3 in mice, and the deletion of MFG-E8 aggravated the disorder of autophagic flux (P < 0.05).
Ferroptosis is a type of cell death closely related to autophagy[38]. To investigate the potential role of ferroptosis in liver injury caused by SAP, we examined the expression levels of two biomarkers of ferroptosis, namely, prostaglandin-endoperoxide synthase 2 (PTGS2) and GPX4. The results showed that SAP resulted in a slight increase in PTGS2 and a significant decrease in GPX4 in the livers of wild-type mice. In MFG-E8-deficient mice, SAP resulted in a significant increase in PTGS2 and almost no GPX4 expression (P < 0.05, Figure 3D and E). Ferroptosis is characterized by iron accumulation, lipid peroxidation and antioxidant system damage[18,29,39], therefore, we detected the expression levels of MDA and GSH. As expected, MFG-E8 deficiency aggravated the elevated MDA levels in the liver and increased GSH consumption (Figure 3F and G). Furthermore, SAP caused an increase in oxygen free radical products in mouse liver tissue, and mfge8 knockdown also increased the accumulation of reactive oxygen species products (Figure 3H).
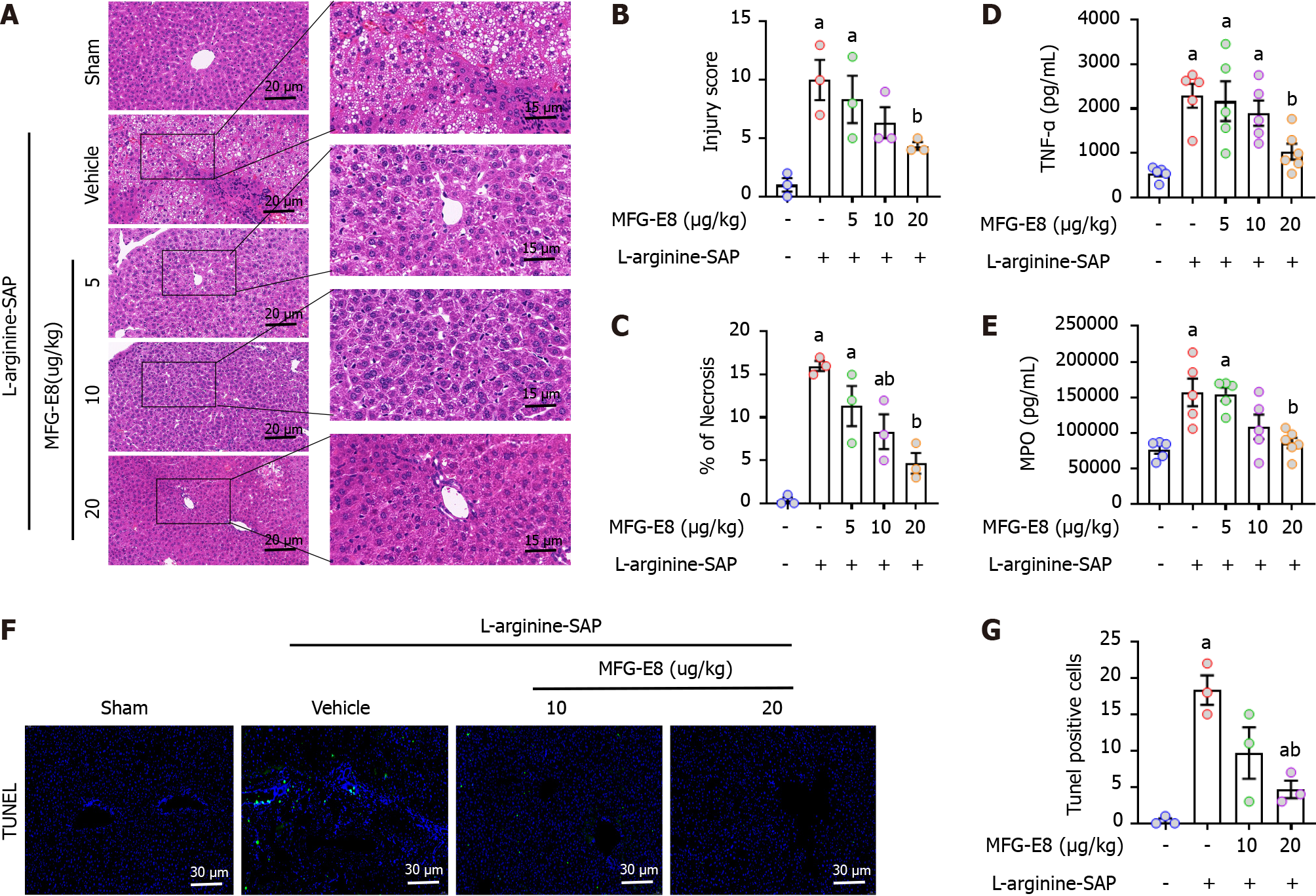
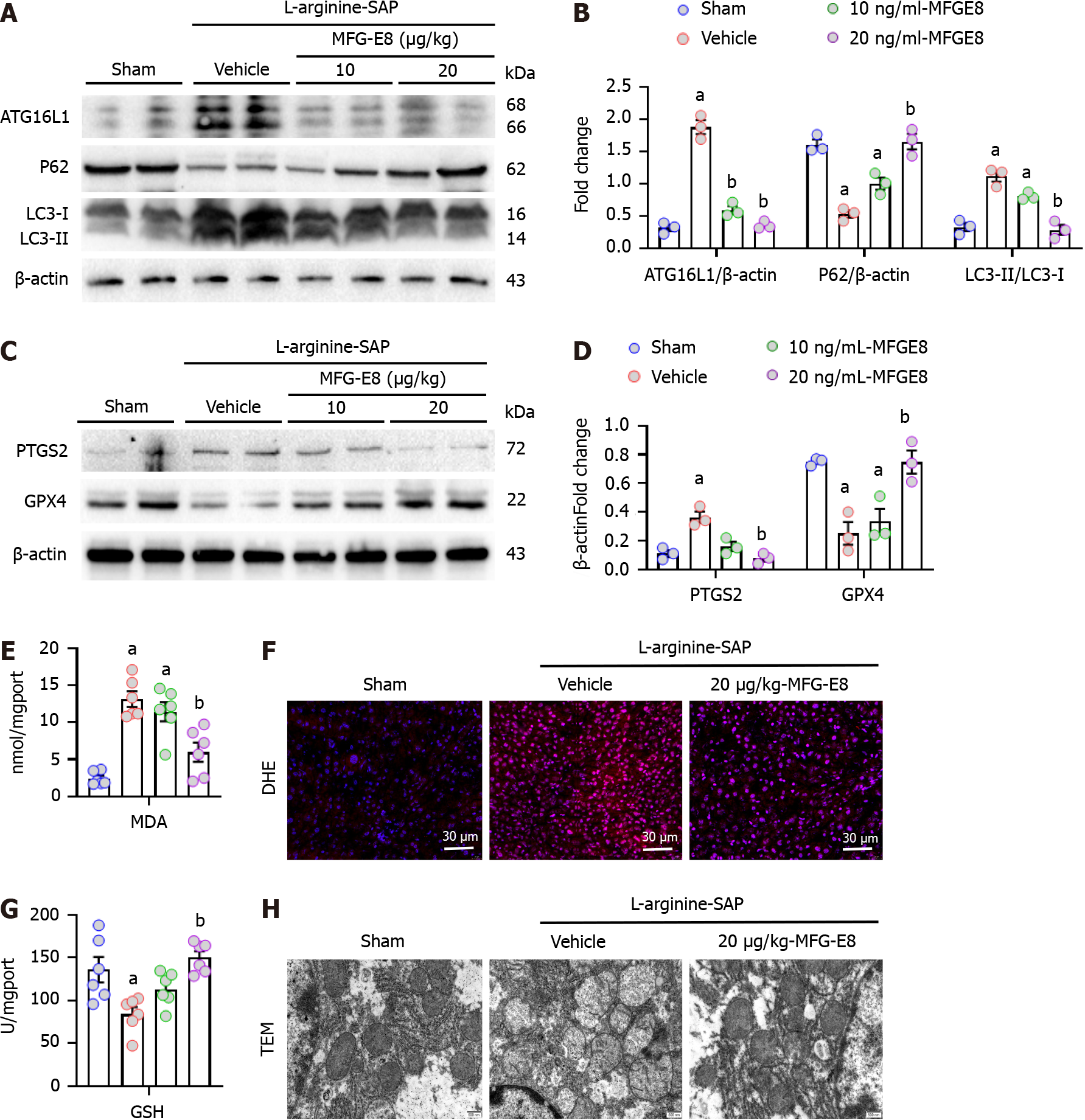
To further investigate the potential protective effect of MFG-E8 on liver injury caused by SAP, recombinant MFG-E8 (5, 10, or 20 μg/kg) was administered 2 h after the second injection of L-arginine. Similar to the results shown in Figure 2A, L-SAP resulted in extensive liver damage in mice with a visual field of hepatic vacuolation and hepatic lobules barely discernible under light microscopy (Figure 4A). Intraperitoneal injection of recombinant MFG-E8 not only restored the changes in liver microstructure (P < 0.05, Figure 4A-C) but also reduced the levels of TNF-α and MPO in the liver parenchyma (P < 0.05, Figure 4D and E). The beneficial effects of MFG-E8 were dose dependent. Similarly, 10 μg/kg or 20 μg/kg MFG-E8 reduced the number of TUNEL-positive cells by 45% (P > 0.05) or 85% (P < 0.05), respectively (Figure 4F and G). Intraperitoneal injection of MFG-E8 also alleviated liver damage caused by cerulein + LPS-induced SAP in a dose-dependent manner (P < 0.05, Supplementary Figure 1A-C).
As expected, 10 μg/kg (low dose) exogenous MFG-E8 partially restored the expression levels of proteins associated with autophagic flux (P > 0.05, Figure 5A and B) and ferroptosis (P > 0.05, Figure 5C and D) in damaged hepatocytes, while 20 μg/kg MFG-E8 almost completely restored the expression levels of these proteins (P < 0.05, Figure 5A-D). The restoration of MDA and GSH production as well as the repair of reactive oxygen species level in hepatocytes also indicated that exogenous MFG-E8 reduced iron accumulation, lipid peroxidation and antioxidant system damage of liver cells in a dose-dependent manner (P < 0.05, Figure 5E-G). We then observed morphological changes in mitochondria under electron microscopy. SAP results in increased electron density of the mitochondrial matrix and destruction of the mitochondrial crest and mitochondrial membrane in hepatocytes, and 20 μg/kg MFG-E8 also relieved these mitochondrial morphological abnormalities (Figure 5H). This evidence suggested that exogenous MFG-E8 supplementation may alleviate liver injury and promote hepatocyte homeostasis in experimental SAP.
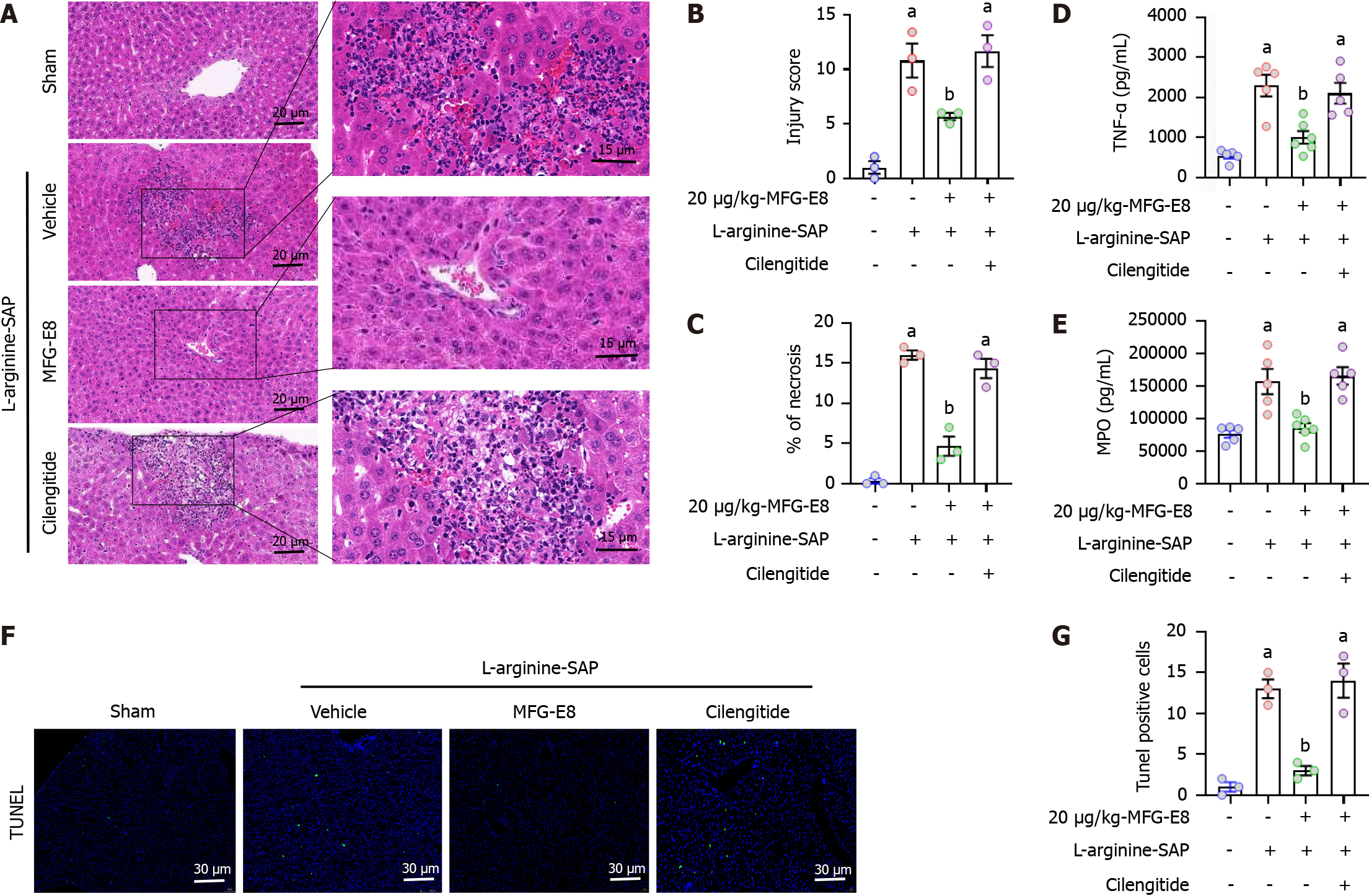
Previous evidence suggests that MFG-E8 is involved in signal transduction mainly by binding the αVβ3 and αVβ5 integrins[23,26,40]. To investigate whether the protective effect of MFG-E8 on the liver is also αVβ3/5 integrin-mediated, we utilized cilengitide, a specific αVβ3/5 integrin inhibitor, to block the possible target of MFG-E8.
H&E staining and damage quantification showed that cilengitide counterbalanced the protective effect of MFG-E8 on the liver of SAP mice (P < 0.05, Figure 6A-C). Similarly, TNF-α (P < 0.05, Figure 6D) and MPO (P < 0.05, Figure 6E) in liver were also increased after cilengitide addition, reaching almost the same level as those in vehicle-treated mice (P > 0.05). The number of TUNEL-positive cells significantly increased after cilengitide was added and even reached 1.1 times that in vehicle-treated mice (P > 0.05, Figure 6F and G).
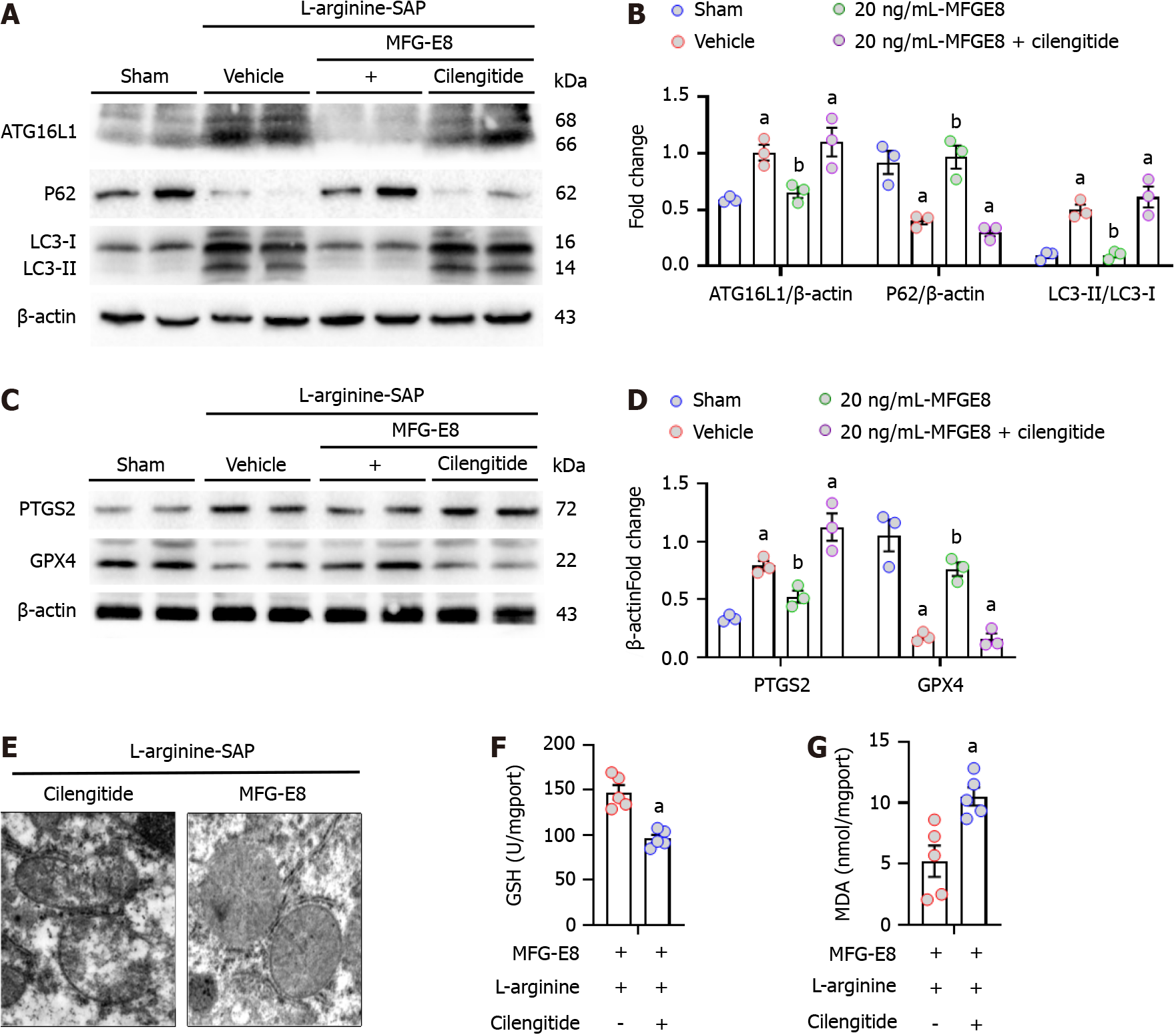
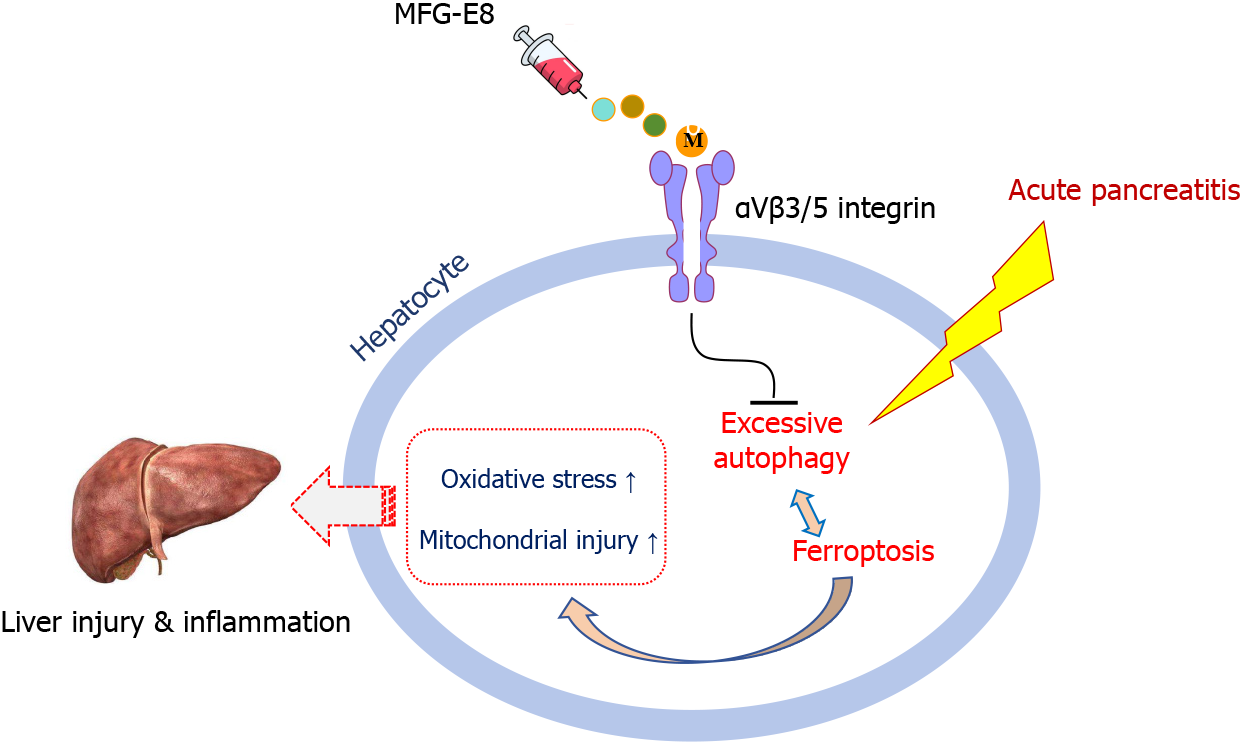
We next explored whether the restoration of autophagic flux and inhibition of ferroptosis by MFG-E8 were also mediated by integrins αVβ3/5. As shown in Figure 7A-D, after the addition of cilengitide, the effect of exogenous MFG-E8 on ATG16L1, P62, LC3 and GPX4 almost disappeared, and the expression level of PTGS2 was approximately 1.2 times that of vehicle-treated mice (P < 0.05). Moreover, iron accumulation, mitochondrial ultramorphological abnormalities, lipid peroxidation and damage to the antioxidant system, which are the signature events of ferroptosis, were also intensified by the addition of cilengitide (P < 0.05, Figure 7E-G). These results suggested that MFG-E8 alleviates liver damage caused by SAP while inhibiting autophagic flux disorder and the exacerbation of ferroptosis through integrins αVβ3/5 (Figure 8).
In the present study, we found that the lack of MFG-E8 aggravated liver damage and the imbalance of hepatocyte homeostasis caused by SAP. Intraperitoneal injection of exogenous MFG-E8 restored excessive autophagy, reduced ferroptosis and alleviated liver injury by acting on integrins αVβ3/5. Moreover, the protective effect of exogenous MFG-E8 on the liver was dose dependent.
Autophagy flux disorder mediates intracellular dysfunction in a variety of pathological conditions[41,42]. As the site of synthesis, metabolism and secretion of various substances necessary for life, the active intracellular environment of hepatocytes is easily affected by excessive autophagy, resulting in homeostasis disorder[43,44]. The formation of autophagic vesicles requires a necessary pair of ubiquitin-like coupling systems, namely, ATG12-ATG5 and ATG8 (LC3)-phosphatidylethanolamine (LC3-PE)[45]. ATG16L1 provides a functional link between these two key autophagy ubiquitin-like coupling systems. ATG16L1 binds ATG5 of the ATG12-ATG5 connector to form an 800 kDa polymeric complex[46]. The ATG12-ATG-5-ATG16L1 complex is located in the preautophagosome membrane where it identifies the site of LC3 lipidation and catalyzes the reaction required for mature autophagosome formation[47,48].
Genome-wide association scans have revealed mutations in ATG16L1, a gene linked to Crohn’s disease[49]. Mice abnormal expression of the ATG16L1 coiled-helix domain show impaired autophagosome formation and increased inflammatory cytokines, consistent with their role in inflammatory disease pathogenesis. Suballele ATG16L1 mice also show autophagy defects and intestinal Pan cell dysfunction similar to that found in Crohn’s disease[50]. In the present study, we found that the lack of MFG-E8 aggravated the abnormal expression of ATG16L1 in hepatocytes caused by SAP and worsened the disorder of autophagic flux (abnormal expression of P62 and LC3). Exogenous MFG-E8 restored liver ATG16L1, P62 and LC3 levels in a dose-dependent manner. These results suggested that the moderating effect of MFG-E8 on the SAP-induced hepatic inflammatory response (increased TNF-α and MPO) may be achieved by restoring ATG16L1 and alleviating excessive autophagy.
Recent evidence suggests that ferroptosis plays a critical role in the development of nonneoplastic liver disease[51]. Ferroptosis is a new type of iron-dependent programmed cell death that is different from apoptosis, cell necrosis and autophagy. The main mechanism of ferroptosis is that under the action of divalent iron or ester oxygenase, unsaturated fatty acids with high expression on the cell membrane are catalyzed to produce lipid peroxidation, thus inducing cell death[18,52]. In addition, decreased expression of antioxidant systems (glutathione GSH and GPX4) has also been observed with ferroptosis[53]. The effect of MFG-E8 on ferroptosis has not been reported in previous studies. In the present study, we found for the first time a possible link between MFG-E8 and ferroptosis in liver cells. It is possible that the protective effect of MFG-E8 on liver injury induced by SAP is also induced by alleviating ferroptosis, which is similar to the protective effect of (+)-clausenamide on the liver by alleviating ferroptosis as reported by Wang et al[54].
PTGS2, also known as cyclooxygenase-2, is a biomarker of ferroptosis similar to GPX4[29]. Ferroptosis is a form of programmed cell death induced by iron-dependent lipid peroxidation. GPX4 prevents rust by converting lipid hydroperoxides into nontoxic lipid alcohols[55]. The present study showed that oxidative stress in hepatocytes caused by lipid peroxidation resulted in mitochondrial membrane structure destruction. Electron microscopy showed that the mitochondria of hepatocytes of SAP mice showed significant swelling or even rupture, and iron ion deposition appeared in mitochondria. The use of exogenous MFG-E8 alleviates oxidative stress and restores mitochondrial morphology in hepatocytes, which is consistent with our recent findings on SAP. Moreover, MFG-E8 activates the focal adhesion kinase (FAK) - signal transduction and transcriptional activation factor 3 (STAT3) signaling pathway and alleviates the extent of mitochondrial damage during SAP. Together, these results indicate that MFG-E8 plays a crucial role in maintaining cellular homeostasis, such as mitochondrial function, lipid oxidation reactions and iron ion metabolism. However, the association of these cellular homeostasis and specific signaling pathways involved in MFG-E8 need to be further explored.
Our previous studies have shown that MFG-E8 maintains cellular homeostasis by alleviating ER stress in pancreatic exocrine acinar cells. The beneficial effects of MFG-E8 are mediated through activating the αVβ3/5 integrin-FAK-STAT3 signaling pathway[28]. The present study also explored the potential role of the αVβ3/5 integrin-FAK-STAT3 signaling pathway in the liver. However, the results showed that inhibition of MFG-E8 binding to integrins αVβ3/5 effectively antagonized the protective effect of MFG-E8 on SAP-induced liver injury, while blocking the FAK-STAT3 signaling pathway had almost no effect on the effect of MFG-E8 in the liver. Elucidating the specific molecular mechanism of the protective effect of MFG-E8 on hepatocytes is our future research direction.
The present study had several deficiencies, and additional studies are required. Due to the lack of clinical samples, we were unable to validate these results in patients with AP. TUNEL fluorescence staining showed that MFG-E8 also reduced SAP-induced apoptosis of liver cells. It remains unknown whether ferroptosis or apoptosis plays a more important role in SAP-induced liver death. Moreover, the role of MFG-E8 in ferroptosis has not been previously reported, and excessive autophagy can lead to ferroptosis[13,56]. Therefore, whether MFG-E8 directly regulates the occurrence of ferroptosis or indirectly alleviates ferroptosis by improving the homeostasis of autophagy flux remains to be explored further. Furthermore, in this study, we discussed the possible role of exogenous MFG-E8 in improving impaired liver function by binding integrin αVβ3/5. However, whether the intracellular MFG-E8 affects the liver injury caused by SAP and the specific mechanism remain obscure. Next, we will construct hepatocyte-specific mfge8 gene modified mice (knockout or over-expression), and combine with in vitro experiments to further clarify the possible role of intracellular MFG-E8 in SAP-liver injury.
MFG-E8 alleviates excessive autophagy, inhibits ferroptosis in hepatocytes, and protects the liver from damage in SAP. The beneficial effects of MFG-E8 appears to be mediated through activating the αVβ3/5 integrin. These findings may provide a new perspective to reveal the role of MFG-E8 in SAP.
Liver injury is common in severe acute pancreatitis (SAP). Excessive autophagy often leads to an imbalance of homeostasis in hepatocytes, which induces lipid peroxidation and mitochondrial iron deposition and ultimately leads to ferroptosis. Our previous study found that milk fat globule epidermal growth factor 8 (MFG-E8) alleviates acinar cell damage during SAP via binding to αvβ3/5 integrins. MFG-E8 also seems to mitigate pancreatic autophagy in chronic pancreatitis. Whether MFG-E8 also alleviates damaged liver cells in SAP through a similar mechanism is unknown.
This study is to identify whether MFG-E8 could alleviate SAP induced liver injury by restoring the abnormal autophagy flux and alleviate mitochondrial damage like it in acute or chronic pancreatitis.
This study aims to investigate the role of MFG-E8 in SAP-related liver injury.
Of the 134 AP patients (age ≥ 18 years) were included in this study. AP was diagnosed according to the International Atlanta Symposium on Acute Pancreatitis. SAP was induced in mice by 2 hly intraperitoneal injections of 4.0 g/kg L-arginine or 7 hly injections of 50 μg/kg cerulein plus lipopolysaccharide. mfge8-knockout mice were used to study the effect of MFG-E8 deficiency on SAP-induced liver injury. Cilengitide, a specific αvβ3/5 integrin inhibitor, was used to investigate the possible mechanism of MFG-E8.
Serum MFG-E8 concentration is negatively correlated with inflammatory severity in AP patients. MFG-E8 deficiency aggravated SAP-induced liver injury in mice, enhanced autophagy flux in hepatocyte, and worsened the degree of ferroptosis. Exogenous MFG-E8 reduced SAP-induced liver injury in a dose-dependent manner. Mechanistically, MFG-E8 mitigated excessive autophagy and inhibited ferroptosis in liver cells. Cilengitide abolished MFG-E8’s beneficial effects in SAP-induced liver injury.
Our findings suggested MFG-E8 acts as an endogenous protective mediator in SAP-induced liver injury. MFG-E8 alleviates the excessive autophagy and inhibits ferroptosis in hepatocytes by binding to integrin αVβ3/5.
These findings may provide a new perspective to reveal the role of MFG-E8 in SAP and its regulation of homeostasis in damaged liver cells.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leowattana W, Thailand S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4304] [Article Influence: 358.7] [Reference Citation Analysis (44)] |
| 2. | Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 813] [Article Influence: 81.3] [Reference Citation Analysis (1)] |
| 3. | Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:479-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 511] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 4. | Mandalia A, Wamsteker EJ, DiMagno MJ. Recent advances in understanding and managing acute pancreatitis. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Piao X, Sui X, Liu B, Cui T, Qi Z. Picroside II Improves Severe Acute Pancreatitis-Induced Hepatocellular Injury in Rats by Affecting JAK2/STAT3 Phosphorylation Signaling. Biomed Res Int. 2021;2021:9945149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Habtezion A, Gukovskaya AS, Pandol SJ. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology. 2019;156:1941-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 7. | Yang CJ, Chen J, Phillips AR, Windsor JA, Petrov MS. Predictors of severe and critical acute pancreatitis: a systematic review. Dig Liver Dis. 2014;46:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Kong L, Zhang H, Lu C, Shi K, Huang H, Zheng Y, Wang Y, Wang D, Wang H, Huang W. AICAR, an AMP-Activated Protein Kinase Activator, Ameliorates Acute Pancreatitis-Associated Liver Injury Partially Through Nrf2-Mediated Antioxidant Effects and Inhibition of NLRP3 Inflammasome Activation. Front Pharmacol. 2021;12:724514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1833] [Article Influence: 166.6] [Reference Citation Analysis (0)] |
| 10. | Jiang W, Chen X, Ji C, Zhang W, Song J, Li J, Wang J. Key Regulators of Autophagosome Closure. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 867] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 12. | Hamaoui D, Subtil A. ATG16L1 functions in cell homeostasis beyond autophagy. FEBS J. 2022;289:1779-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ 3rd, Kang R, Tang D. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1630] [Article Influence: 181.1] [Reference Citation Analysis (0)] |
| 14. | Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D. Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol. 2020;66:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 707] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 15. | Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 612] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 16. | Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H, AlQudsy LHH, Shang P. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 383] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 17. | Stockwell BR, Jiang X, Gu W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020;30:478-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 774] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 18. | Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1891] [Cited by in RCA: 2384] [Article Influence: 596.0] [Reference Citation Analysis (0)] |
| 19. | Yu Y, Jiang L, Wang H, Shen Z, Cheng Q, Zhang P, Wang J, Wu Q, Fang X, Duan L, Wang S, Wang K, An P, Shao T, Chung RT, Zheng S, Min J, Wang F. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood. 2020;136:726-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 20. | Capelletti MM, Manceau H, Puy H, Peoc'h K. Ferroptosis in Liver Diseases: An Overview. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 21. | Wei S, Bi J, Yang L, Zhang J, Wan Y, Chen X, Wang Y, Wu Z, Lv Y, Wu R. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin Transl Med. 2020;10:e173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 22. | An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, Park JY, Park SY, Kim JH, Do BR, Han C, Park HK, Kim OH, Song MJ, Kim SJ. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology. 2017;152:1174-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 23. | Yang C, Hayashida T, Forster N, Li C, Shen D, Maheswaran S, Chen L, Anderson KS, Ellisen LW, Sgroi D, Schmidt EV. The integrin alpha(v)beta(3-5) ligand MFG-E8 is a p63/p73 target gene in triple-negative breast cancers but exhibits suppressive functions in ER(+) and erbB2(+) breast cancers. Cancer Res. 2011;71:937-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Miksa M, Amin D, Wu R, Jacob A, Zhou M, Dong W, Yang WL, Ravikumar TS, Wang P. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 2008;22:743-748. [PubMed] |
| 25. | Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 996] [Cited by in RCA: 1066] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 26. | Aziz M, Yang WL, Corbo LM, Chaung WW, Matsuo S, Wang P. MFG-E8 inhibits neutrophil migration through αvβ₃-integrin-dependent MAP kinase activation. Int J Mol Med. 2015;36:18-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Ren Y, Liu W, Zhang L, Zhang J, Bi J, Wang T, Wang M, Du Z, Wang Y, Wu Z, Lv Y, Meng L, Wu R. Milk fat globule EGF factor 8 restores mitochondrial function via integrin-medicated activation of the FAK-STAT3 signaling pathway in acute pancreatitis. Clin Transl Med. 2021;11:e295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Ren Y, Liu W, Zhang J, Bi J, Fan M, Lv Y, Wu Z, Zhang Y, Wu R. MFG-E8 Maintains Cellular Homeostasis by Suppressing Endoplasmic Reticulum Stress in Pancreatic Exocrine Acinar Cells. Front Cell Dev Biol. 2021;9:803876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Zhao C, Yu D, He Z, Bao L, Feng L, Chen L, Liu Z, Hu X, Zhang N, Wang T, Fu Y. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic Biol Med. 2021;175:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 30. | Zhang L, Tian R, Yao X, Zhang XJ, Zhang P, Huang Y, She ZG, Li H, Ji YX, Cai J. Milk Fat Globule-Epidermal Growth Factor-Factor 8 Improves Hepatic Steatosis and Inflammation. Hepatology. 2021;73:586-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, Nam DH, Hau P, Weyerbrock A, Taphoorn MJ, Shen CC, Rao N, Thurzo L, Herrlinger U, Gupta T, Kortmann RD, Adamska K, McBain C, Brandes AA, Tonn JC, Schnell O, Wiegel T, Kim CY, Nabors LB, Reardon DA, van den Bent MJ, Hicking C, Markivskyy A, Picard M, Weller M; European Organisation for Research and Treatment of Cancer (EORTC); Canadian Brain Tumor Consortium; CENTRIC study team. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 781] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 32. | Li Y, Gao Q, Shu X, Xiao L, Yang Y, Pang N, Luo Y, He J, Zhang L, Wu J. Antagonizing αvβ3 Integrin Improves Ischemia-Mediated Vascular Normalization and Blood Perfusion by Altering Macrophages. Front Pharmacol. 2021;12:585778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Gong JD, Qi XF, Zhang Y, Li HL. Increased admission serum cold-inducible RNA-binding protein concentration is associated with prognosis of severe acute pancreatitis. Clin Chim Acta. 2017;471:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Ren YF, Wang MZ, Bi JB, Zhang J, Zhang L, Liu WM, Wei SS, Lv Y, Wu Z, Wu RQ. Irisin attenuates intestinal injury, oxidative and endoplasmic reticulum stress in mice with L-arginine-induced acute pancreatitis. World J Gastroenterol. 2019;25:6653-6667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Liu R, Cui J, Sun Y, Xu W, Wang Z, Wu M, Dong H, Yang C, Hong S, Yin S, Wang H. Autophagy deficiency promotes M1 macrophage polarization to exacerbate acute liver injury via ATG5 repression during aging. Cell Death Discov. 2021;7:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Gendy AM, Elnagar MR, Allam MM, Mousa MR, Khodir AE, El-Haddad AE, Elnahas OS, Fayez SM, El-Mancy SS. Berberine-loaded nanostructured lipid carriers mitigate warm hepatic ischemia/reperfusion-induced lesion through modulation of HMGB1/TLR4/NF-κB signaling and autophagy. Biomed Pharmacother. 2022;145:112122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Brennan A, Layfield R, Long J, Williams HEL, Oldham NJ, Scott D, Searle MS. An ALS-associated variant of the autophagy receptor SQSTM1/p62 reprograms binding selectivity toward the autophagy-related hATG8 proteins. J Biol Chem. 2022;298:101514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Liu Z, Ma C, Wang Q, Yang H, Lu Z, Bi T, Xu Z, Li T, Zhang L, Zhang Y, Liu J, Wei X, Li J. Targeting FAM134B-mediated reticulophagy activates sorafenib-induced ferroptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2022;589:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 39. | Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 4097] [Article Influence: 1024.3] [Reference Citation Analysis (0)] |
| 40. | Pan D, Wu W, Zuo G, Xie X, Li H, Ren X, Kong C, Zhou W, Zhang Z, Waterfall M, Chen S. Sphingosine 1-phosphate receptor 2 promotes erythrocyte clearance by vascular smooth muscle cells in intraplaque hemorrhage through MFG-E8 production. Cell Signal. 2022;98:110419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 41. | Levine B, Kroemer G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell. 2019;176:11-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 2041] [Article Influence: 340.2] [Reference Citation Analysis (0)] |
| 42. | Yao RQ, Ren C, Xia ZF, Yao YM. Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy. 2021;17:385-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 43. | Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14:170-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 396] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 44. | Ruart M, Chavarria L, Campreciós G, Suárez-Herrera N, Montironi C, Guixé-Muntet S, Bosch J, Friedman SL, Garcia-Pagán JC, Hernández-Gea V. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J Hepatol. 2019;70:458-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 45. | Lin TY, Chan HH, Chen SH, Sarvagalla S, Chen PS, Coumar MS, Cheng SM, Chang YC, Lin CH, Leung E, Cheung CHA. BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy. 2020;16:1296-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 46. | Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 595] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 47. | Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1659] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 48. | Lystad AH, Carlsson SR, de la Ballina LR, Kauffman KJ, Nag S, Yoshimori T, Melia TJ, Simonsen A. Distinct functions of ATG16L1 isoforms in membrane binding and LC3B lipidation in autophagy-related processes. Nat Cell Biol. 2019;21:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 49. | Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Günther S, Prescott NJ, Onnie CM, Häsler R, Sipos B, Fölsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1519] [Cited by in RCA: 1453] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 50. | Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, Stone CD, Brunt EM, Xavier RJ, Sleckman BP, Li E, Mizushima N, Stappenbeck TS, Virgin HW 4th. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1256] [Cited by in RCA: 1242] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 51. | Mao L, Zhao T, Song Y, Lin L, Fan X, Cui B, Feng H, Wang X, Yu Q, Zhang J, Jiang K, Wang B, Sun C. The emerging role of ferroptosis in non-cancer liver diseases: hype or increasing hope? Cell Death Dis. 2020;11:518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 52. | Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1070] [Cited by in RCA: 2076] [Article Influence: 207.6] [Reference Citation Analysis (0)] |
| 53. | Lei P, Bai T, Sun Y. Mechanisms of Ferroptosis and Relations With Regulated Cell Death: A Review. Front Physiol. 2019;10:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 386] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 54. | Wang M, Liu CY, Wang T, Yu HM, Ouyang SH, Wu YP, Gong HB, Ma XH, Jiao GL, Fu LL, Wu QS, Kurihara H, Li YF, Shen T, He RR. (+)-Clausenamide protects against drug-induced liver injury by inhibiting hepatocyte ferroptosis. Cell Death Dis. 2020;11:781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 55. | Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1498] [Cited by in RCA: 2395] [Article Influence: 399.2] [Reference Citation Analysis (0)] |
| 56. | Zhang Z, Guo M, Li Y, Shen M, Kong D, Shao J, Ding H, Tan S, Chen A, Zhang F, Zheng S. RNA-binding protein ZFP36/TTP protects against ferroptosis by regulating autophagy signaling pathway in hepatic stellate cells. Autophagy. 2020;16:1482-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 330] [Article Influence: 66.0] [Reference Citation Analysis (0)] |