Published online Dec 7, 2024. doi: 10.3748/wjg.v30.i45.4771
Revised: September 27, 2024
Accepted: October 29, 2024
Published online: December 7, 2024
Processing time: 72 Days and 19.6 Hours
Acute pancreatitis (AP) is a common acute gastrointestinal disorder affecting approximately 20% of patients with systemic inflammatory responses that may cause pancreatic and peripancreatic fat necrosis. This condition often progresses to multiple organ failure, significantly increasing morbidity and mortality. Oxidative stress, characterized by an imbalance between the body’s reactive oxygen species (ROS) and antioxidants, activates the inflammatory signaling pathways. Although the pathogenesis of AP is not fully understood, ROS are increasingly recognized as critical in the disease's progression and development. Modulating the oxidative stress pathway has shown efficacy in mitigating the progression of AP. Despite numerous basic studies examining this pathway, comprehensive reviews of recent research remain sparse. This systematic review offers an in-depth examination of the critical role of oxidative stress in the pathogenesis and progression of AP and evaluates the therapeutic potential of antioxidant interventions in its management.
Core Tip: Acute pancreatitis (AP) is a common acute gastrointestinal disorder. Reactive oxygen species significantly impact the progression and development of AP, and modulation of the oxidative stress pathway can effectively mitigate this condition. This systematic review examines the pivotal role of oxidative stress in the pathogenesis and progression of AP and the therapeutic potential of anti-oxidative stress interventions in its management.
- Citation: Xia CC, Chen HT, Deng H, Huang YT, Xu GQ. Reactive oxygen species and oxidative stress in acute pancreatitis: Pathogenesis and new therapeutic interventions. World J Gastroenterol 2024; 30(45): 4771-4780
- URL: https://www.wjgnet.com/1007-9327/full/v30/i45/4771.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i45.4771
Acute pancreatitis (AP) manifests as an acute abdominal condition triggered by abnormal activation of pancreatic enzymes, which produce digestive effects on the pancreas and nearby organs. It is marked by a local inflammatory response in the pancreas, potentially leading to organ dysfunction[1]. Abdominal pain is the predominant symptom, accompanied by low-to-moderate fever, nausea, and vomiting[1]. The incidence of AP is 1.60 deaths (95% confidence interval [CI]: 0.85-1.58) and 33.74 cases (95%CI: 23.33-48.81) per 100000 person-years according to estimates[1], with an observed increase over time[2]. The pathogenesis of AP has been extensively studied, identifying excessive alcohol intake and gallstone-induced ductal obstruction as primary factors, alongside hypertriglyceridemia, medications, and autoimmune disorders as additional contributors[3]. The clinical presentation of AP often includes interstitial swelling, vacuole accumulation, and inflammatory cell infiltration[4]. The severity and complications of AP are closely linked to the extent of infection and pancreatic necrosis[5]. While most cases are mild and resolve within weeks, responding well to conservative treatment, approximately 20% of patients develop systemic inflammatory responses with pancreatic and peripancreatic fat necrosis, leading to significant morbidity and mortality[6,7]. Studies have shown that the relative risk of mortality doubles in cases with concurrent organ failure and necrosis, highlighting the severe risk in critical cases of AP[8]. Obesity is a prognostic factor for AP severity, with complications being more severe in obese individuals[9,10].
The pathogenesis of AP remains incompletely elucidated. Current research suggests that the initiation of AP is primarily due to the abnormal activation of trypsinogen[11], occurring within the acinar cell[12]. Various pathological signals, such as calcium (Ca2+) overload[13], impaired autophagy[14,15], endoplasmic reticulum (ER) stress[16], and oxidative stress[17], contribute to the disease onset and progression. Oxidative stress is critical in the early stages of AP, as reactive oxygen species (ROS) modify signal transduction pathways susceptible to redox control and directly cause oxidative damage[18].
The pathogenesis and development of AP are significantly influenced by ROS. An intriguing finding is that neutrophils derived from patients with AP exhibit increased generation of ROS[19]. ROS appear to be primarily produced by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs), with nuclear factor kappa B (NF-κB) acting as the main target in redox signaling in AP[20,21]. The expression of inflammatory factors regulated by NF-κB, as well as NF-κB activation, are extremely important in the development and exacerbation of AP. Several studies have confirmed that modulation of this pathway can reduce inflammation, such as the use of a caffeine-free extract from green tea, which mitigates inflammation by attenuating NF-κB activation and suppressing pro-inflammatory responses[22]. Additionally, ascorbic acid and N-acetyl cysteine have shown favorable effects in reducing acute inflammation in mice with AP[23]. This article offers a thorough analysis of the vital function ROS plays in the etiology and development of AP, and the therapeutic potential of anti-oxidative stress interventions in managing AP.
In biology and medicine, ROS encompass various oxidants generated by molecular oxygen (O2-). They belong to a group of reactive species, classified based on the reactive atom involved, including reactive nitrogen species (RNS), sulfur, carbon, and others. These species have been shown to cause oxidative changes in biological macromolecules through redox reactions, playing a crucial role in redox signaling and biological functions[24].
ROS consists of molecules produced through redox reactions or electronic excitation from O2-. These molecules exhibit a wide range of chemical reactivities, with second-order rate constants towards specific targets spanning up to 11 orders of magnitude[25,26]. The group includes oxygen-containing free radicals such as superoxide anion and hydroxyl radicals. Given that ‘ROS’ serves as a broad yet chemically vague term in redox biology studies, researchers recommend identifying the precise species being investigated. When specific details are unavailable, it is advisable to refer to these compounds as “oxidants”[27]. In this review, we specifically examine hydrogen peroxide (H2O2) and O2-, both of which are well-documented in terms of their functions.
ROS is a byproduct of normal cellular metabolism, playing complex roles in signal transduction pathways and maintaining tissue homeostasis[24]. Most ROS are produced by the mitochondrial respiratory chain and perform essential cellular functions, such as regulating redox-sensitive transcription factors and interacting with various molecules[28].
Exposure to various inflammatory stimuli leads to the excessive production of highly reactive compounds such as RNS and ROS, both within and outside tissues or cells. The inability of tissues or cells to efficiently remove these highly reactive molecules disrupts oxidative balance, leading to oxidative stress imbalance, resulting in cell death and tissue damage. At supra-physiological concentrations, ROS exhibit non-specific reactivity towards proteins, lipids, and other cellular components. Additionally, they generate further reactive species, potentially causing hazardous effects[28].
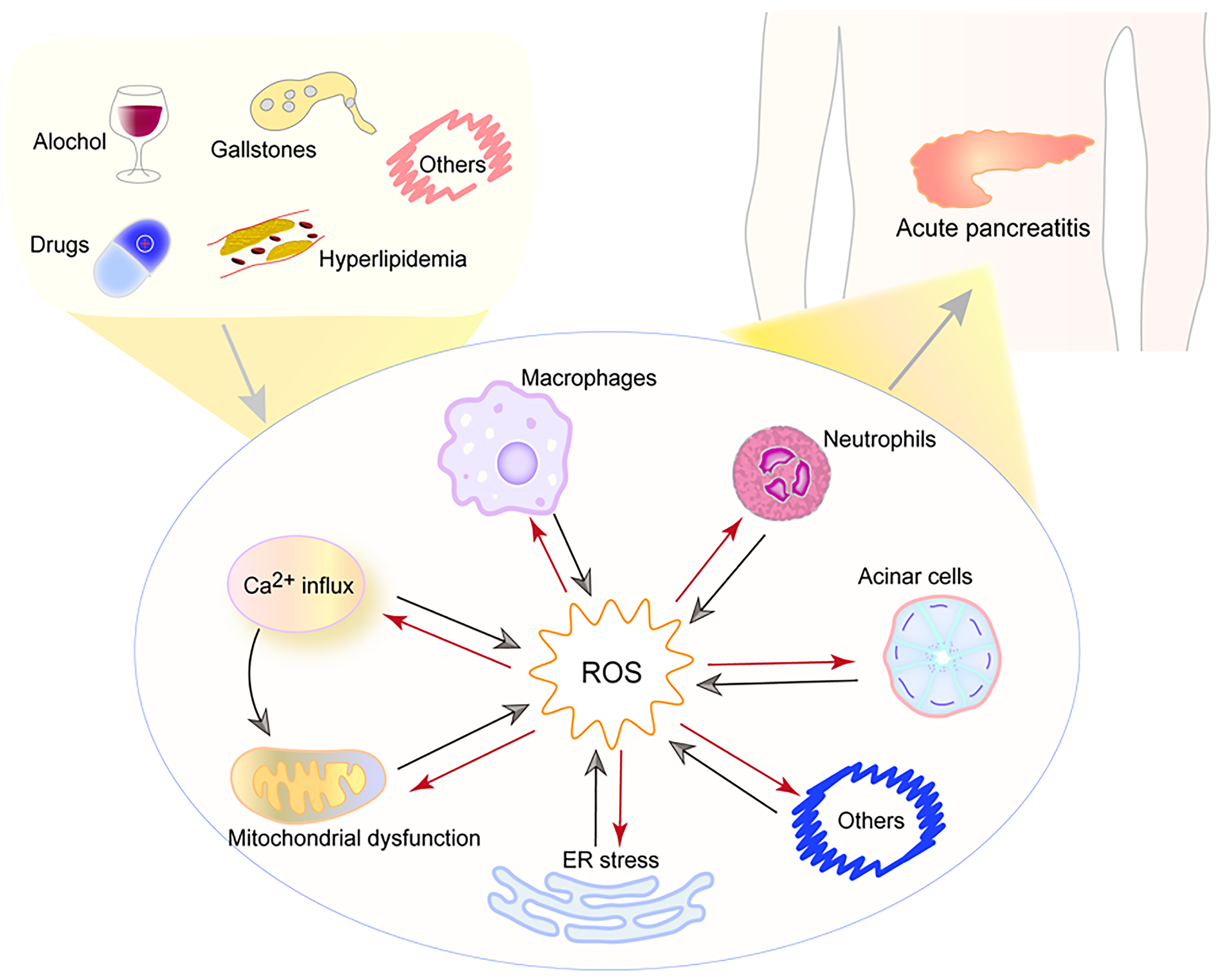
Oxidative stress occurs when the body’s antioxidant system and the generation of ROS are out of balance, which can activate multiple signaling pathways. Moreover, ROS act as critical signaling molecules, regulating both inflammatory cascades and the aggregation of inflammatory cells, such as neutrophils, significantly contributing to pancreatic and systemic injury, ROS can also cause pathophysiological changes such as mitochondrial dysfunction, ER stress, and Ca2+ influx[29]. In turn, these pathophysiological changes also stimulate ROS production and aggravate cell and tissue damage, which is shown in Figure 1. Studies have identified malondialdehyde (MDA) as a primary biomarker for oxidative stress, while superoxide dismutase (SOD) activity and total antioxidant capacity indicate the body’s capability to neutralize free radicals[30,31]. Other key biomarkers include glutathione peroxidase (GSH-Px) and 4-hydroxynonenal[30].
During AP, immune cells trigger an acute inflammatory response by secreting various cytokines, which activate inflammation in multiple disease processes[12,32,33]. Influenced by inflammatory mediators, such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and IL-6, endothelial permeability increases, microcirculation is impaired, and highly reactive molecules such as ROS are produced. These ROS directly damage biomolecules, including DNA, proteins, and lipids, and affect transcription and protein expression. They also react with unsaturated fatty acids in cell membranes, creating harmful byproducts such as MDA, leading to membrane damage and cell death. This cascade exacerbates disease progression and complications[18,34].
Telek et al[35] demonstrated in situ the formation of oxygen free radicals during rat and human pancreatitis using the cerium group trapping method[35], and identified that ROS sources vary according to the experimental models of AP. In mild AP induced by cerulein, radical formation is primarily linked to activated neutrophil infiltration, while in necrotic AP induced by retrograde taurocholate injection, radical production is associated with the conversion of xanthine dehydrogenase (XDH) to xanthine oxidase (XO). Due to its significant capacity to generate large amounts of free radicals upon activation, the role of XDH has been extensively studied in ischemia-reperfusion situations and various diseases. In AP, this enzyme plays a critical role in both localized and systemic responses. Granell et al[36] suggested that increased α-amylase activity in AP disrupts the connection between endothelial cell glycoproteins and XDH, leading to significant enzyme release into the circulation, and proteolytic enzymes in plasma may activate XO[36]. Consequently, XO-induced pulmonary oxidative damage can be mitigated by administering oxypurinol, a water-soluble derivative of allopurinol[37]. This intervention reduces leukocyte infiltration and myeloperoxidase (MPO) activity in experimental AP[38].
Being the primary non-protein thiol in the cells of mammals, reduced glutathione (GSH) is essential for antioxidant function. It remains in balance with oxidized GSH (GSSG), and a reliable measure of oxidative stress is the ratio of GSSG to GSH, indicating the delicate balance in prooxidant processes and cellular antioxidant activity[39], and the pancreas showed a substantial reduction in reduced GSH and an increase in tissue and plasma lipid peroxidation during experimental AP, and one feature of AP observed in its early stages is a reduction of GSH in the pancreatic tissue, GSH monoethyl ester pretreatment has been demonstrated to alleviate the inflammation in AP by raising GSH levels in the pancreas[17]. However, rats with AP that received L-buthionine-(S,R)-sulfoximine to inhibit GSH synthesis had higher pancreatic necrosis and worse survival rates. Importantly, the shift from mitogen activated protein (MAP) to stress-activated protein (SAP) may be facilitated by the depletion of GSH[40].
Oxidative stress is commonly observed in patients with AP, as clinical studies have shown. Antioxidant enzyme levels, including SOD and GSH-PX, significantly decrease, while levels of MPO, MDA, and protein carbonyl groups - all markers of oxidative damage - significantly increase[41,42]. Furthermore, a correlation has been observed between MDA levels and the incidence of AP complications[43] and multiple organ dysfunction[44]. The severity of AP correlates with changes in superoxide radicals and lipid peroxide levels in the blood, as multiple studies have indicated[45]. Additionally, indicators of oxidative stress are correlated with serum phospholipase A2 and plasma polymorphonuclear elastin activity, serving as prognostic indicators for AP[17]. Moreover, vitamin A may affect the expression of multiple molecular pathways linked to cytoprotection and antioxidative defense[46].
During the development of AP, oxidative stress regulates multiple crucial cellular processes and is essential to the development of pancreatic injury. Increasing levels of ROS induced by the oxidant menadione result in a higher rate of apoptotic cell death, indicating that ROS formation influences the fate of pancreatic acinar cells[47]. ROS in tissues primarily originate from NOX[48]. The superoxides generated by NOX within cells, such as H2O2, hold essential roles in several routes of signaling, including NF-κB activation, and apoptosis signal-regulated kinase 1[49]. Redox processes involving protein kinases and phosphatases may regulate NF-κB activation, as these enzymes are susceptible to oxidation[50]. Consequently, IκB phosphorylation caused by ROS will cause it to separate from NF-κB dimers, which will then activate NF-κB[51]. Accordingly, pro-oxidants such as H2O2 can activate NF-κB in different kinds of cells. On the contrary, research has revealed that antioxidants can inhibit the activation of NF-κB[52]. The degree to which oxidative stress activates NF-κB depends on the ROS-producing mechanism[53]. Research has shown significant upregulation of NOX expression and activity in the pancreatic tissues of mice with experimental AP[54]. Some cell types, such as mouse alveolar epithelial cells, have no effect on NF-κB in response to H2O2, which has also been observed to reduce NF-κB nuclear translocation[55]. Oxidation of the IκB kinase (IKK) complex's cysteine residues leads to reduced activation of NF-κB by H2O2 through inhibition of IKK activity[55]. Furthermore, oxidation can directly target NF-κB, particularly p50, reducing its DNA-binding capacity[50,51]. The new research demonstrates that neutrophil extracellular traps, a unique neutrophil defense system function, contribute to pancreatic inflammation and distant organ damage during pancreatitis[56]. In pancreatic acinar cells, ROS-induced apoptosis can be facilitated by NOX, which also alters the production of inflammatory factors such as IL-6[20]. Research shows that controlling NOX activity through NAD(P)H quinone dehydrogenase 1’s enzymatic modification of the cellular NADPH:NADP+ ratio prevents AP[57].
ROS plays a far greater part in the inflammatory cascade than was previously thought. Recent research has indicated that mitochondrial ROS function as signals, through both inflammasome-dependent and inflammasome-independent pathways, causing the generation of pro-inflammatory cytokines[58,59]. Large cytoplasmic multiprotein complexes called inflammasomes connect the proteolytic activity of certain cytokines to the detection of microbial compounds that damage cells, damage-associated molecular patterns secreted by the necrotic cells, or the metabolic stress that nucleotide oligomerization domain-like receptors cause[60,61]. Inhibition of mitochondrial ROS prevents lipopolysaccharide-triggered activation of the MAP kinase (MAPK) signaling pathway and subsequent synthesis of TNF-α and IL-6 through a mechanism that does not involve inflammasomes[62]. Notably, the nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing (NLRP) inflammasome and mitochondrial ROS are necessary to release mitochondrial DNA (mt-DNA), which enhances inflammation activation[63]. It is interesting to note that blocking autophagy or mitophagy causes damaged ROS-producing mitochondria to accumulate and discharge mt-DNA into the cytoplasm, which activates the NLRP3 inflammasome and caspase-1 as a result[59]. Importantly, both the NLRP inflammasome and mitochondrial ROS are essential for the release of mt-DNA, which amplifies inflammatory responses[63]. Protein phosphatases have a significant part in redox signaling during the inflammatory reaction because oxidative stress affects their activity. Since thiols in protein phosphatases can be oxidized reversibly to create intramolecular disulfide bridges or sulfenyl-amide linkages, which inactivate the enzymes, they are crucial indicators of the redox condition of cells[64,65]. Thus, oxidative stress promotes MAPK activation by deactivating protein phosphatases. ROS-induced MAPK activation is primarily mediated by MKP inhibition through various signaling pathways[66]. For example, TNF-α-induced mitochondrial ROS converts the catalytic cysteine of MAPK phosphatases (MPKs) to sulfenic acid, causing MKP oxidation and inhibition, which results in prolonged c-Jun N-terminal kinase activation.
According to the theory of Ca2+ overload, damage to cell membranes caused by AP leads to an influx of extracellular Ca2+ into the cells, causing excessive Ca2+ accumulation. This excess Ca2+ further impairs mitochondrial function, inhibits energy-dependent Ca2+ pumps, and prematurely activates digestive enzymes, Causing a severe inflammatory response. However, ROS can intensify Ca2+ overload through various channels, compromising its barrier function[13]. It has also been shown that pancreatic acinar cell mitochondria produce ROS when exposed to bile acids, elevating mitochondrial Ca2+ in both humans and mice[67].
In addition to ROS's extensive role in the etiology of AP, RNS are essential to its advancement. The destructive impacts of highly reactive RNS are comparable to those of ROS, as they directly affect biomolecules such as lipids or proteins, triggering pro-inflammatory signaling cascades that ultimately lead to immune responses[68]. The progression of AP results in the induction of inducible nitric oxide synthase (iNOS) in tissues, producing a significant amount of highly RNS, including NO[69]. Under normal conditions, the endogenous production of NO is beneficial; however, uncontrolled and excessive NO can cause tissue damage[70,71].
The etiology and progression of AP are profoundly influenced by oxidative stress, and modulating this pathway can significantly mitigate the progression of this condition. Within the body, there are two types of molecules with antioxidant properties: One includes antioxidant enzyme molecules, such as SOD, and catalase; the other comprises non-enzymatic antioxidant molecules including melatonin, vitamins, carotenoids, and α-lipoic acid. Research on AP treatment with antioxidants has focused primarily on these two antioxidant systems.
Although the specific regulators of this inflammasome machinery remain unidentified, pancreatic cell damage mediated by the NLRP3 inflammasome is the fundamental pathophysiology of AP. Membrane-associated RING-CH 9 is part of the family of MARCH-type E3 ubiquitin ligases, which facilitate the polyubiquitination of crucial immunological components to regulate innate immunity. Studies show that MARCH9 attenuates pancreatic cell damage mediated by the NLRP3 inflammasome by targeting the ubiquitination and degradation of NOX-2, thereby reducing ROS production and NLRP3 inflammasome activation[72]. Research has also found that high-density lipoprotein (HDL) derived from humans protects against cell death both in vitro and in vivo. The mimic peptide D4F has been identified as a highly effective protector against AP. Reduction of gasdermin D (GSDMD) and constitutive caspase-1, or NLRP3 conditional on acinar cells, could counteract the protective benefits of HDL, indicating that HDL could prevent acinar cell pyroptosis and benefit AP treatment[73]. Therefore, the GSDMD pyroptosis signaling pathway and the NLRP3 inflammasome are potential therapeutic targets for directed AP treatment[74] (Table 1).
| Ref. | Treatment | Journal | Year | Model |
| Gao et al[74] | NLRP3 and caspase-1 inhibitors | British Journal of Pharmacology Vol | 2021 | Caerulein-induced AP in mice |
| Rong et al[38] | Xanthine oxidase | Acta Pharmaceutica Sinica. B | 2024 | L-arginine-induced AP, caerulein-induced AP, and retrograde injection of 3.5% NaT induced AP in mice |
| Zheng et al[75] | Allopurinol | Pancreas | 2008 | Post-ERCP pancreatitis in patients |
| Romagnuolo et al[76] | Allopurinol | Clinical Gastroenterology and Hepatology | 2008 | Post-ERCP pancreatitis in patients |
| Katsinelos et al[77] | Allopurinol | Gastrointestinal Endoscopy | 2005 | Post-ERCP pancreatitis in patients |
| Li et al[78] | Deoxyarbutin | Free Radical Research | 2022 | Retrograde injection of 3.5% NaT induced AP in mice |
| Cuzzocrea et al[79] | iNOS inhibitors | Shock | 2002 | Caerulein-induced AP in mice |
| He et al[80] | Sulfiredoxin-1 | Cell Death & Disease | 2021 | Caerulein-induced AP in mice |
| Zhao et al[81] | Melatonin | European Journal of Pharmacology | 2024 | Caerulein plus LPS induced AP in mice |
| Yuan et al[82] | Vitamin B12 | Oxidative Medicine and Cellular Longevity | 2021 | Retrograde injection of 3.5% NaT induced AP in mice |
| Gui et al[83] | Vitamin C | Journal of Inflammation Research | 2023 | Retrograde injection of 3.5% NaT induced AP in mice |
| Sadeghi et al[84] | Vitamin C | Clinical Endoscopy | 2023 | Post-ERCP pancreatitis in patients |
Rong et al[38] reported that AP was alleviated by XO inhibition through hypoxia inducible factor 1 alpha-regulated lactate dehydrogenase A and NLRP3 signaling pathways. However, the translation of these findings to clinical practice is debated. Zheng et al[75] and Romagnuolo et al[76] stated that the use of allopurinol for prophylactic purposes in preventing and treating pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP) did not result in a reduction in the incidence of pancreatitis or hyperamylasemia. However, other studies suggest that allopurinol may effectively lower the risk of postoperative pancreatitis and shorten hospital stays[77], highlighting the need for further well-designed, large-scale clinical studies.
Li et al[78] found that deoxyarbutin, a tyrosinase inhibitor, significantly reduces acinar necrosis and ROS production, thereby preserving normal mitochondrial function and lessening pancreatic damage in AP mice. Cuzzocrea et al[79] observed that mice deficient in iNOS showed less severe pancreatic inflammation induced by cerulein compared to wild-type mice, suggesting a possible function of iNOS inhibitors as therapeutic agents for AP. Additionally, Chvanov et al[69] demonstrated significant improvement in experimental acute pancreatic inflammation following the administration of highly selective inducible iNOS inhibitors, which effectively inhibited iNOS activity. Sulfiredoxin-1 (Srxn1) is an endogenous small molecule protein selected evolutionarily for its antioxidant and anti-apoptotic functions. He et al[80] showed that Srxn1 alleviates inflammation in AP mice through regulation of the ROS/ER stress/cathepsin B axis, underscoring its potential as a protective target for AP. Zhao et al[81] found that melatonin mitigates AP by effectively blocking cell death in pancreatic tissues. Importantly, melatonin also reduces damage to distant organs, such as the lungs and liver.
The therapeutic role of vitamins has drawn more attention in recent years (Table 2). The study conducted by Yuan et al[82] demonstrated that vitamin B12 has a beneficial effect on AP by activating cystathionine-β synthase, a crucial coenzyme involved in GSH synthesis. This mechanism significantly reduces oxidative stress and improves mitochondrial dysfunction. Gui et al[83] determined that in rats with SAP, high-dose vitamin C reduces pancreatic necrosis by inhibiting platelet activation through the C-X-C motif chemokine 12/C-X-C chemokine receptor type 4 pathway. A clinical study by Sadeghi et al[84] indicated that rectal indomethacin and vitamin C injection together decreased the occurrence of pancreatitis caused by ERCP.
| Ref. | Treatment | Journal | Year | Animal model |
| Yang et al[85] | Triptolide | International Journal of Molecular Sciences | 2022 | Caerulein-induced AP in mice |
| Ren et al[86] | Danshensu | Frontiers In Immunology | 2018 | Caerulein-induced AP in mice |
| Rong et al[87] | Resveratrol | Oxidative Medicine and Cellular Longevity | 2021 | Retrograde injection of 3.5% NaT induced AP in rats |
| Yang et al[88] | Chaiqin Chengqi Decoction | Frontiers In Pharmacology | 2022 | Obesity-related AP in mice |
| Xia et al[89] | Emodin | Inflammation | 2019 | Retrograde injection of 3.5% NaT induced AP in rats |
| Seo et al[90] | Quercetin 3-O-xyloside | Phytomedicine | 2019 | Lipopolysaccharide and cerulein-induced cellular AP |
| Xie et al[91] | Prussian blue nanozyme | Theranostics | 2021 | Caerulein-induced AP in mice |
| Zhou et al[92] | Celastrol | Molecular Pharmaceutics | 2019 | Retrograde injection of 3.5% NaT induced AP in rats |
Various components derived from traditional Chinese medicine have shown significant antioxidant effects. Triptolide reduces AP by suppressing the NF-κB-mediated signal pathway and activating the nuclear factor erythroid 2-related factor-mediated expression of antioxidant genes[85]. The natural product Danshensu also reduces AP through a comparable mechanism[86]. Resveratrol, as a natural antioxidant, eases AP by activating the sirtuin 1-forkhead box protein O1 axis to reduce disturbances in microcirculation[87]. The therapeutic mechanism of Chaiqin Chengqi Decoction (CQCQD) in mice with alcohol-induced obesity-related AP was identified by Yang et al[88] through transcriptomic analysis. Specifically, CQCQD helps reduce pancreatic necrosis and ease systemic inflammation in visceral adipose tissue and pancreatic tissue by downregulating the phosphoinositide 3-kinase/Akt signaling pathway. Emodin, a key bioactive compound derived from rhubarb, lessens the severity of AP due to its potent antioxidative and anti-inflammatory properties[89]. Quercetin 3-O-xyloside, noted for its strong anti-pancreatitis activity, can work as a therapeutic agent to treat AP or as a beneficial functional ingredient in health-promoting foods. It achieves this by enhancing apoptotic cell death and reducing the production of intracellular ROS and the ER stress response[90].
Additionally, the rapid development of nanomaterials provides innovative strategies for antioxidant therapy in AP. Prussian blue nanomaterials are known for their activity as oxygen scavengers. Xie et al[91] demonstrated that Prussian blue-mediated nanomaterials can effectively improve AP in mice by inhibiting the Toll-like receptor/NF-κB signaling pathway. Furthermore, these nanomaterials exhibit stable and safe biological properties. Zhou et al[92] developed a nanomaterial encapsulated by neutrophil cell membrane and coated with triptolide, utilizing the inherent targeting capability of neutrophils to penetrate the blood-pancreatic barrier in pancreatic inflammation. This design ensures specific delivery to pancreatic tissue, enhancing the anti-inflammatory and antioxidative effects of the material and effectively mitigating disease progression.
ROS are byproducts of normal cellular metabolism, playing complex roles in signal transduction pathways and are essential for maintaining tissue homeostasis. When AP occurs, it induces oxidative stress. This imbalance can trigger the activation of signaling pathways responsible for inflammatory responses in the body, leading to various inflammatory cascades, resulting in inflammatory progression and organ tissue damage. During the development of AP, oxidative stress regulates several critical cellular processes and influences the development of pancreatic injury. Modulating the oxidative stress pathway can effectively mitigate the progression of this condition. The body contains two categories of molecules that exert antioxidant effects: Antioxidant enzyme molecules and non-enzymatic antioxidant molecules. Antioxidant therapy is an effective treatment strategy for AP, research on AP treatment with antioxidants primarily focuses on these two types of antioxidant systems, which have recently been the subject of significant basic research. However, current research primarily utilizes animal models of AP, which are far removed from clinical application. Indeed, current treatments for AP still lack key therapeutic drugs, and oxidative stress plays a critical role in the disease's development; thus, the potential for drug intervention treatment is considerable. Meanwhile, it has been observed that current studies do not sufficiently report possible adverse events caused by antioxidant drugs used in the treatment of pancreatitis, which should not be overlooked as antioxidants may disrupt the normal ROS signaling pathway. Clearly, further research is required to develop redox-based medicines, which will need to carefully identify and quantify the species involved in signaling, as well as determine the locations and rates at which they are produced within cells. There is still much to learn about the relationship between the redox state and antioxidants and gene expression. This necessitates more precise oxidant definitions and the development of improved analytical instruments, such as real-time imaging techniques that can identify the production of oxidants locally and the signal transduction they induce.
| 1. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 528] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 2. | Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen AA, Swain M, Buie M, Underwood FE, Kaplan GG. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022;162:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 363] [Article Influence: 121.0] [Reference Citation Analysis (1)] |
| 3. | Wang GJ, Gao CF, Wei D, Wang C, Ding SQ. Acute pancreatitis: etiology and common pathogenesis. World J Gastroenterol. 2009;15:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (5)] |
| 4. | Mederos MA, Reber HA, Girgis MD. Acute Pancreatitis: A Review. JAMA. 2021;325:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 499] [Article Influence: 124.8] [Reference Citation Analysis (1)] |
| 5. | Xue P, Deng LH, Zhang ZD, Yang XN, Wan MH, Song B, Xia Q. Infectious complications in patients with severe acute pancreatitis. Dig Dis Sci. 2009;54:2748-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 585] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 7. | Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort HC, Bruno MJ; Dutch Pancreatitis Study Group. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 289] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 8. | Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 564] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 9. | Chen SM, Xiong GS, Wu SM. Is obesity an indicator of complications and mortality in acute pancreatitis? An updated meta-analysis. J Dig Dis. 2012;13:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Zhu Y, Huang Y, Sun H, Chen L, Yu H, Shi L, Xia W, Sun X, Yang Y, Huang H. Novel anthropometric indicators of visceral obesity predict the severity of hyperlipidemic acute pancreatitis. Lipids Health Dis. 2024;23:120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Mayerle J, Sendler M, Hegyi E, Beyer G, Lerch MM, Sahin-Tóth M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019;156:1951-1968.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 12. | Saluja A, Dudeja V, Dawra R, Sah RP. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology. 2019;156:1979-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 13. | Pallagi P, Madácsy T, Varga Á, Maléth J. Intracellular Ca(2+) Signalling in the Pathogenesis of Acute Pancreatitis: Recent Advances and Translational Perspectives. Int J Mol Sci. 2020;21:4005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Mareninova OA, Hermann K, French SW, O'Konski MS, Pandol SJ, Webster P, Erickson AH, Katunuma N, Gorelick FS, Gukovsky I, Gukovskaya AS. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340-3355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Ohmuraya M, Yamamura K. Autophagy and acute pancreatitis: a novel autophagy theory for trypsinogen activation. Autophagy. 2008;4:1060-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Han X, Li B, Bao J, Wu Z, Chen C, Ni J, Shen J, Song P, Peng Q, Wan R, Wang X, Wu J, Hu G. Endoplasmic reticulum stress promoted acinar cell necroptosis in acute pancreatitis through cathepsinB-mediated AP-1 activation. Front Immunol. 2022;13:968639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 17. | Pérez S, Pereda J, Sabater L, Sastre J. Redox signaling in acute pancreatitis. Redox Biol. 2015;5:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Yu JH, Kim H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J Gastroenterol. 2014;20:17324-17329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 19. | Susak YM, Dirda OO, Fedorchuk OG, Tkachenko OA, Skivka LM. Infectious Complications of Acute Pancreatitis Is Associated with Peripheral Blood Phagocyte Functional Exhaustion. Dig Dis Sci. 2021;66:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Yu JH, Lim JW, Kim H, Kim KH. NADPH oxidase mediates interleukin-6 expression in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol. 2005;37:1458-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Kim H. Inhibitory mechanism of lycopene on cytokine expression in experimental pancreatitis. Ann N Y Acad Sci. 2011;1229:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Babu BI, Malleo G, Genovese T, Mazzon E, Di Paola R, Crisafulli C, Caminiti R, Siriwardena AK, Cuzzocrea S. Green tea polyphenols ameliorate pancreatic injury in cerulein-induced murine acute pancreatitis. Pancreas. 2009;38:954-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Eşrefoğlu M, Gül M, Ates B, Batçioğlu K, Selimoğlu MA. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J Gastroenterol. 2006;12:259-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE, Murphy MP, Yamamoto M, Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23:499-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 819] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 25. | Hawkins CL, Davies MJ. Detection, identification, and quantification of oxidative protein modifications. J Biol Chem. 2019;294:19683-19708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 262] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 26. | Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 2270] [Article Influence: 283.8] [Reference Citation Analysis (0)] |
| 27. | Yang Z, Min Z, Yu B. Reactive oxygen species and immune regulation. Int Rev Immunol. 2020;39:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 28. | Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 2878] [Article Influence: 575.6] [Reference Citation Analysis (0)] |
| 29. | Xu Y, Song J, Gao J, Zhang H. Identification of Biomarkers Associated with Oxidative Stress and Immune Cells in Acute Pancreatitis. J Inflamm Res. 2024;17:4077-4091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Bopanna S, Nayak B, Prakash S, Shalimar, Mahapatra SJ, Garg PK. Increased oxidative stress and deficient antioxidant levels may be involved in the pathogenesis of idiopathic recurrent acute pancreatitis. Pancreatology. 2017;17:529-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Zheng X, Li L, Zhu Y, Huang X, Zhang Y, Yu B, He W, Lv N. Superoxide Dismutase Predicts Persistent Circulation Failure and Mortality in the Early Stage of Acute Pancreatitis. Dig Dis Sci. 2020;65:3551-3557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, Partecke LI, Döring P, Gukovsky I, Gukovskaya AS, Wagh PR, Lerch MM, Mayerle J. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology. 2018;154:704-718.e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 33. | Sendler M, Dummer A, Weiss FU, Krüger B, Wartmann T, Scharffetter-Kochanek K, van Rooijen N, Malla SR, Aghdassi A, Halangk W, Lerch MM, Mayerle J. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut. 2013;62:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 34. | Armstrong JA, Cash NJ, Ouyang Y, Morton JC, Chvanov M, Latawiec D, Awais M, Tepikin AV, Sutton R, Criddle DN. Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift. J Biol Chem. 2018;293:8032-8047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Telek G, Scoazec JY, Chariot J, Ducroc R, Feldmann G, Roz C. Cerium-based histochemical demonstration of oxidative stress in taurocholate-induced acute pancreatitis in rats. A confocal laser scanning microscopic study. J Histochem Cytochem. 1999;47:1201-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Granell S, Bulbena O, Genesca M, Sabater L, Sastre J, Gelpi E, Closa D. Mobilization of xanthine oxidase from the gastrointestinal tract in acute pancreatitis. BMC Gastroenterol. 2004;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Granell S, Serrano-Mollar A, Folch-Puy E, Navajas D, Farre R, Bulbena O, Closa D. Oxygen in the alveolar air space mediates lung inflammation in acute pancreatitis. Free Radic Biol Med. 2004;37:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Rong J, Han C, Huang Y, Wang Y, Qiu Q, Wang M, Wang S, Wang R, Yang J, Li X, Hu C, Chen Z, Deng L, Huang W, Xia Q, Du D. Inhibition of xanthine oxidase alleviated pancreatic necrosis via HIF-1α-regulated LDHA and NLRP3 signaling pathway in acute pancreatitis. Acta Pharm Sin B. 2024;14:3591-3604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 39. | Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865-1879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1163] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 40. | Pereda J, Escobar J, Sandoval J, Rodríguez JL, Sabater L, Pallardó FV, Torres L, Franco L, Viña J, López-Rodas G, Sastre J. Glutamate cysteine ligase up-regulation fails in necrotizing pancreatitis. Free Radic Biol Med. 2008;44:1599-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Park BK, Chung JB, Lee JH, Suh JH, Park SW, Song SY, Kim H, Kim KH, Kang JK. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol. 2003;9:2266-2269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 42. | Thangaraj KR, Priyadarshini SJ, Qureshi IN, Joseph AJ, Balasubramanian KA, Ramachandran A. Plasma Citrulline, Glycans, and Hydrogen Sulfide in Patients With Acute Pancreatitis: Possible Markers of Intestinal Damage. Pancreas. 2016;45:e27-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Hernández V, Miranda M, Pascual I, Sanchiz V, Almela P, Añón R, Cuadrado E, Sanz MI, Mínguez M, Mora F, Romero FJ, Benages A. Malondialdehyde in early phase of acute pancreatitis. Rev Esp Enferm Dig. 2011;103:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Shi C, Andersson R, Zhao X, Wang X. Potential role of reactive oxygen species in pancreatitis-associated multiple organ dysfunction. Pancreatology. 2005;5:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Pădureanu V, Florescu DN, Pădureanu R, Ghenea AE, Gheonea DI, Oancea CN. Role of antioxidants and oxidative stress in the evolution of acute pancreatitis (Review). Exp Ther Med. 2022;23:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 46. | Burzyński J, Fichna J, Tarasiuk A. Putative molecular targets for vitamin A in neutralizing oxidative stress in acute and chronic pancreatitis - a systematic review. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 47. | Criddle DN, Gillies S, Baumgartner-Wilson HK, Jaffar M, Chinje EC, Passmore S, Chvanov M, Barrow S, Gerasimenko OV, Tepikin AV, Sutton R, Petersen OH. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J Biol Chem. 2006;281:40485-40492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 281] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 48. | Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 293] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 49. | Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal. 2009;11:1249-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Pantano C, Reynaert NL, van der Vliet A, Janssen-Heininger YM. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 2006;8:1791-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 51. | Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. 2005;7:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 450] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 52. | Akter KA, Sharma S, Sifat AE, Zhang Y, Patel DK, Cucullo L, Abbruscato TJ. Metformin ameliorates neuroinflammatory environment for neurons and astrocytes during in vitro and in vivo stroke and tobacco smoke chemical exposure: Role of Nrf2 activation. Redox Biol. 2024;75:103266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 53. | Bonizzi G, Piette J, Merville MP, Bours V. Cell type-specific role for reactive oxygen species in nuclear factor-kappaB activation by interleukin-1. Biochem Pharmacol. 2000;59:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 54. | Chan YC, Leung PS. Angiotensin II type 1 receptor-dependent nuclear factor-kappaB activation-mediated proinflammatory actions in a rat model of obstructive acute pancreatitis. J Pharmacol Exp Ther. 2007;323:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Korn SH, Wouters EF, Vos N, Janssen-Heininger YM. Cytokine-induced activation of nuclear factor-kappa B is inhibited by hydrogen peroxide through oxidative inactivation of IkappaB kinase. J Biol Chem. 2001;276:35693-35700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Ishqi HM, Ali M, Dawra R. Recent advances in the role of neutrophils and neutrophil extracellular traps in acute pancreatitis. Clin Exp Med. 2023;23:4107-4122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Shen A, Kim HJ, Oh GS, Lee SB, Lee S, Pandit A, Khadka D, Sharma S, Kim SY, Choe SK, Yang SH, Cho EY, Shim H, Park R, Kwak TH, So HS. Pharmacological stimulation of NQO1 decreases NADPH levels and ameliorates acute pancreatitis in mice. Cell Death Dis. 2018;10:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 490] [Cited by in RCA: 601] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 59. | Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3253] [Cited by in RCA: 4258] [Article Influence: 283.9] [Reference Citation Analysis (0)] |
| 60. | Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1443] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 61. | Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 521] [Cited by in RCA: 493] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 62. | Anderson P. Post-transcriptional control of cytokine production. Nat Immunol. 2008;9:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 63. | Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2499] [Cited by in RCA: 2410] [Article Influence: 172.1] [Reference Citation Analysis (0)] |
| 64. | Welsh CL, Madan LK. Protein Tyrosine Phosphatase regulation by Reactive Oxygen Species. Adv Cancer Res. 2024;162:45-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 65. | Yu ZH, Zhang ZY. Regulatory Mechanisms and Novel Therapeutic Targeting Strategies for Protein Tyrosine Phosphatases. Chem Rev. 2018;118:1069-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 66. | Lee K, Esselman WJ. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic Biol Med. 2002;33:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Booth DM, Murphy JA, Mukherjee R, Awais M, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Petersen OH, Sutton R, Criddle DN. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 68. | Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009;11:135-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 69. | Chvanov M, Petersen OH, Tepikin A. Free radicals and the pancreatic acinar cells: role in physiology and pathology. Philos Trans R Soc Lond B Biol Sci. 2005;360:2273-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Ang AD, Adhikari S, Ng SW, Bhatia M. Expression of nitric oxide synthase isoforms and nitric oxide production in acute pancreatitis and associated lung injury. Pancreatology. 2009;9:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 71. | Ozturk F, Gul M, Esrefoglu M, Ates B. The contradictory effects of nitric oxide in caerulein-induced acute pancreatitis in rats. Free Radic Res. 2008;42:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Lin M, Jin Y, Wang F, Meng Y, Huang J, Qin X, Fan Z. MARCH9 Mediates NOX2 Ubiquitination to Alleviate NLRP3 Inflammasome-Dependent Pancreatic Cell Pyroptosis in Acute Pancreatitis. Pancreas. 2023;52:e62-e69. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Lu Y, Li B, Wei M, Zhu Q, Gao L, Ma N, Ma X, Yang Q, Tong Z, Lu G, Li W. HDL inhibits pancreatic acinar cell NLRP3 inflammasome activation and protect against acinar cell pyroptosis in acute pancreatitis. Int Immunopharmacol. 2023;125:110950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 74. | Gao L, Dong X, Gong W, Huang W, Xue J, Zhu Q, Ma N, Chen W, Fu X, Gao X, Lin Z, Ding Y, Shi J, Tong Z, Liu T, Mukherjee R, Sutton R, Lu G, Li W. Acinar cell NLRP3 inflammasome and gasdermin D (GSDMD) activation mediates pyroptosis and systemic inflammation in acute pancreatitis. Br J Pharmacol. 2021;178:3533-3552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 75. | Zheng M, Chen Y, Bai J, Xin Y, Pan X, Zhao L. Meta-analysis of prophylactic allopurinol use in post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2008;37:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Romagnuolo J, Hilsden R, Sandha GS, Cole M, Bass S, May G, Love J, Bain VG, McKaigney J, Fedorak RN. Allopurinol to prevent pancreatitis after endoscopic retrograde cholangiopancreatography: a randomized placebo-controlled trial. Clin Gastroenterol Hepatol. 2008;6:465-71; quiz 371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Katsinelos P, Kountouras J, Chatzis J, Christodoulou K, Paroutoglou G, Mimidis K, Beltsis A, Zavos C. High-dose allopurinol for prevention of post-ERCP pancreatitis: a prospective randomized double-blind controlled trial. Gastrointest Endosc. 2005;61:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 78. | Li Y, Zhu Y, Li S, Dong Y, Wan C, Yu X, Xin G, Wei Z, Li F, Wang Y, Zhang K, Chen Q, Zhang C, Wen E, Niu H, Huang W. Deoxyarbutin attenuates severe acute pancreatitis via the HtrA2/PGC-1α pathway. Free Radic Res. 2022;56:651-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 79. | Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Centorrino T, Ciccolo A, Van de Loo FA, Britti D, Caputi AP, Thiemermann C. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock. 2002;17:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 80. | He J, Ma M, Li D, Wang K, Wang Q, Li Q, He H, Zhou Y, Li Q, Hou X, Yang L. Sulfiredoxin-1 attenuates injury and inflammation in acute pancreatitis through the ROS/ER stress/Cathepsin B axis. Cell Death Dis. 2021;12:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 81. | Zhao T, Fang R, Ding J, Liu Y, Cheng M, Zhou F, Liu F, Li W, Li S, Jiang K, Shi X, Liu M, Xu B, Zou X, Zhu H, Zhou L. Melatonin ameliorates multiorgan injuries induced by severe acute pancreatitis in mice by regulating the Nrf2 signaling pathway. Eur J Pharmacol. 2024;975:176646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 82. | Yuan J, Wei Z, Xin G, Liu X, Zhou Z, Zhang Y, Yu X, Wan C, Chen Q, Zhao W, Wang X, Dong Y, Chen Z, Chen X, Niu H, Huang W. Vitamin B(12) Attenuates Acute Pancreatitis by Suppressing Oxidative Stress and Improving Mitochondria Dysfunction via CBS/SIRT1 Pathway. Oxid Med Cell Longev. 2021;2021:7936316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 83. | Gui M, Huang J, Sheng H, Chen Y, Yang Z, Ma L, Wang D, Xu L, Sun W, Liu J, Xu Y, Chen E, Zhao B, Mao E. High-Dose Vitamin C Alleviates Pancreatic Necrosis by Inhibiting Platelet Activation Through the CXCL12/CXCR4 Pathway in Severe Acute Pancreatitis. J Inflamm Res. 2023;16:2865-2877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Sadeghi A, Jafari-Moghaddam R, Ataei S, Asadiafrooz M, Abbasinazari M. Role of vitamin C and rectal indomethacin in preventing and alleviating post-endoscopic retrograde cholangiopancreatography pancreatitis: a clinical study. Clin Endosc. 2023;56:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 85. | Yang J, Tang X, Ke X, Dai Y, Shi J. Triptolide Suppresses NF-κB-Mediated Inflammatory Responses and Activates Expression of Nrf2-Mediated Antioxidant Genes to Alleviate Caerulein-Induced Acute Pancreatitis. Int J Mol Sci. 2022;23:1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Ren Z, Li H, Zhang M, Zhao Y, Fang X, Li X, Chen W, Zhang H, Wang Y, Pan LL, Sun J. A Novel Derivative of the Natural Product Danshensu Suppresses Inflammatory Responses to Alleviate Caerulein-Induced Acute Pancreatitis. Front Immunol. 2018;9:2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | Rong Y, Ren J, Song W, Xiang R, Ge Y, Lu W, Fu T. Resveratrol Suppresses Severe Acute Pancreatitis-Induced Microcirculation Disturbance through Targeting SIRT1-FOXO1 Axis. Oxid Med Cell Longev. 2021;2021:8891544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 88. | Yang X, Yao L, Yuan M, Zhang X, Jakubowska MA, Ferdek PE, Dai L, Yang J, Jin T, Deng L, Fu X, Du D, Liu T, Criddle DN, Sutton R, Huang W, Xia Q. Transcriptomics and Network Pharmacology Reveal the Protective Effect of Chaiqin Chengqi Decoction on Obesity-Related Alcohol-Induced Acute Pancreatitis via Oxidative Stress and PI3K/Akt Signaling Pathway. Front Pharmacol. 2022;13:896523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 89. | Xia S, Ni Y, Zhou Q, Liu H, Xiang H, Sui H, Shang D. Emodin Attenuates Severe Acute Pancreatitis via Antioxidant and Anti-inflammatory Activity. Inflammation. 2019;42:2129-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 90. | Seo JY, Pandey RP, Lee J, Sohng JK, Namkung W, Park YI. Quercetin 3-O-xyloside ameliorates acute pancreatitis in vitro via the reduction of ER stress and enhancement of apoptosis. Phytomedicine. 2019;55:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | Xie X, Zhao J, Gao W, Chen J, Hu B, Cai X, Zheng Y. Prussian blue nanozyme-mediated nanoscavenger ameliorates acute pancreatitis via inhibiting TLRs/NF-κB signaling pathway. Theranostics. 2021;11:3213-3228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 92. | Zhou X, Cao X, Tu H, Zhang ZR, Deng L. Inflammation-Targeted Delivery of Celastrol via Neutrophil Membrane-Coated Nanoparticles in the Management of Acute Pancreatitis. Mol Pharm. 2019;16:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |